Ekosistemy, 30: 64–68 (2022) https://ekosystems.cfuv.ru
![]()
УДК 582.374.2
Structure and physiological state of field horsetail tissues
Sytnikov D. M., Kucherik G. V.
Sevastopol state university
Sevastopol, Russia
sytnikov@list.ru
The research studied the ultrastructure and physiological state of the main parenchyma of the rhizome, as well as the chlorenchyma of the vegetative shoots of the field horsetail (Equisetum arvense L.). JSM-6060 LA scanning microscope and JEM-1230 transmission electron microscope (JEOL, Japan) were used for the anatomical and ultrastructural analysis of horsetail tissues. The content of soluble and easily-hydrolyzing carbohydrates was found by calculating the mass fraction of sugars and starch (percentage) to air-dry mass. The content of photosynthetic pigments was identified by extraction of chlorophyll with 96 % ethanol followed by calculation of optical density of the extracts and computing pigment concentration of crude mass (mg/g). Lipid droplets, mitochondria and amyloplasts filled with starch grains were found in the cells of the main parenchyma of the rhizome, which contains maximum amount of starch. Moreover, the study registered groups of functionally active chloroplasts in the cells of chlorenchyme, which contained a high amount of photosynthetic pigments and sugars. The presented data on the structure and physiological indicators of the state of tissues complement the concept of adaptive capabilities of the field horsetail.
Key words: field horsetail, ultrastructure, tissue physiological state.
INTRODUCTION
Field horsetail (Equisetum arvense L.) is a perennial herbaceous plant. Generative (spore-bearing) shoots appear in early spring, at the tops they bear ovoid-cylindrical strobili with sporangia, in which spores are formed. After sporulation, generative shoots mostly die off. Instead, vegetative (assimilating) shoots develop, which reach 15–50 cm in height and consist of 14–15 internodes.
Field horsetail shoots have a pronounced metamerism, the assimilation function here is performed by green stems and branches. The rhizome of the field horsetail of two types – horizontal and vertical, bears leaf sheaths and forms tubers, which serve as a place for deposition of reserve substances, as well as organs of vegetative reproduction (Flora, 1936; Kucheryava et al., 1997; Sytnikov et al., 2014).
Horsetails are characterized by significant morphological and ecological plasticity, in connection with which they are of interest in studying the features of the distribution of pioneer vegetation. They are also indicator plants that characterize environmental conditions, such as the presence of acidic soils.
Field horsetail has a high biological efficiency in the distribution of photosynthesis products, has the ability to accumulate reserve substances both in the aerial part of the plant, and in the rhizome and tubers (Marshall, 1986).
Representatives of the genus are characterized by variability of chemical composition during the year (Plant, 1978; Botirov et al., 2021), thus, changes in the content of sugars, starch, and photosynthetic pigments can serve as indicators of the physiological state of tissues and the adaptive capabilities of horsetail. Previously, we established the leading role of chlorenchyma of the lower branches of vegetative shoots of horsetail in the accumulation of the maximum amount of chlorophyll (Sytnikov et al., 2014).
The purpose of current work is to study the features of the ultrastructure of the assimilating and storage tissues of horsetail, as well as the content of physiologically significant substances in them.
ISSN 2414-4738 Published by V. I. Vernadsky Crimean Federal University, Simferopol
Structure and physiological state of field horsetail tissues
![]()
MATERIALS AND METHODS
In current work, generative (spore-bearing) and vegetative (assimilating) shoots of field horsetail (Equisetum arvense) growing in natural conditions were used. Phenological observations of plants were carried out according to the generally accepted method (Baydeman, 1974) in the period from March to August.
The structural features of tissues were studied using a JSM-6060 LA scanning microscope (JEOL, Japan). Samples frozen at liquid nitrogen temperature were dried at –40 °C in vacuum, then covered with a layer of gold in an ION Sputer JFC-1100 ion sputter (JEOL, Japan).
To study the ultrastructure of horsetail, tissue fragments 1×2 mm in size were taken, which were fixed with a 3 % solution of glutaraldehyde (Serva, USA) in phosphate buffer (pH 7.2) for 2 h. The final fixation was carried out with a 1 % osmium tetroxide solution at room temperature for 3 h. The samples were dehydrated with increasing concentrations of ethanol according to the standard procedure (Furst, 1979) and embedded with a mixture (epon and araldite) of epoxy resins (Serva, USA). Ultrathin sections were obtained on an LKB-3 ultramicrotome (LKB, Sweden) and counterstained with lead citrate according to Reynolds (Gayer, 1974) for 7 min. Tissue sections were examined using a JEM-1230 transmission electron microscope (JEOL, Japan).
The content of soluble and easily hydrolysable carbohydrates was determined according to the described procedure (Methods, 1989). Samples were fixed for 20 min at 105 °C and dried at 60– 65 °C for 4 h. Determination of the content of photosynthetic pigments was carried out by preliminary extraction of chlorophyll with 96 % ethanol, followed by determination of the optical density of the obtained extracts on a spectrophotometer PE 5400UF (Russia) at 665 and 649 nm. After determining the concentration of chlorophylls a and b in the extract (Shlyk, 1975), their content in the studied plant material was calculated using the formula of Wintermans and De Mots, taking into account the extract volume and sample weight (Musienkо et al., 2001).
Statistical processing of the obtained data was carried out according to Dospekhov (1985). The figures and text show %%, arithmetic mean, standard errors and least significant difference (LSD) were determined. The significance of the difference in values was assessed using a 5 % significance level (P≤0.05). The sizes of organelles and cells were calculated from electron micrographs using the UTHSCSA Image Tool 3.0 program.
RESULTS AND DISCUSSION
The surface of the rhizome internodes of the field horsetail is more even in comparison with the surface of the above-ground internodes of its vegetative shoots, and its ridges are weakly expressed. It is known (Plant, 1978) that rhizomes do not have stomata, chlorenchyma, and strands of mechanical tissue of the same type as in above-ground vegetative shoots. Under the epidermis of the rhizome internode lie several layers of more or less thick-walled, not lignified, but impregnated with fat-like substances and silica parenchymal cells, under which, in turn, lie thin-walled cells of the main parenchyma containing starch grains.
Microphotographs of rhizome tissues obtained using a scanning electron microscope clearly showed spherical starch grains. The study of the tissue ultrastructure of field horsetail (fig. 1), obtained using a transmission electron microscope, showed that in the phase of young assimilating shoots (15 cm), the cells of the main parenchyma of the rhizome contained numerous amyloplasts filled with starch grains, lipid drops, and mitochondria with a well-developed cristae system. (see fig. 1a).
The preliminary study of soluble and easily hydrolyzed carbohydrates in the generative period showed that the maximum amount of sugars (15.9 %) and starch (12.9 %) was contained in the field horsetail rhizome during the meristematic shoot phase. In the vegetative period of development of field horsetail (fig. 2), the content of sugars in the rhizome significantly decreased to 7.4 % of the air-dry mass with the development of assimilating shoots. However, the content of starch in the rhizome increased noticeably up to 14.0 % compared to the generative period.
65
Sytnikov D. M., Kucherik G. V.
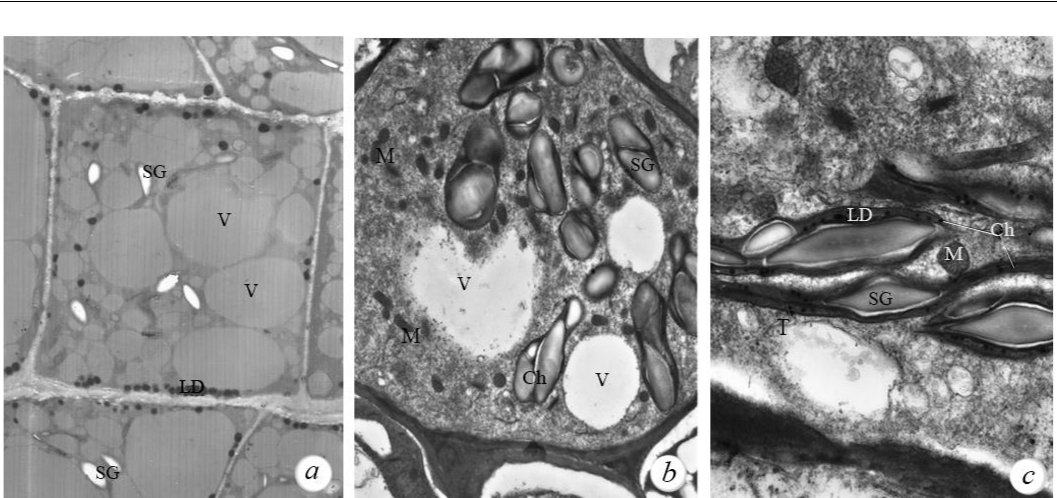
Fig. 1. Cell ultrastructure of storage and assimilating tissues of field horsetail (Equisetum arvense)
a – main rhizome parenchyma (750× magnification); b (3000× magnification) and c (8000× magnification) – internode chlorenchyma. Figure captions: SG – starch grains; LD – lipid drops; V – vacuoles; Ch – chloroplasts; T – tillakoids; M – mitochondria.
A decrease in the amount of starch in the rhizome of field horsetail in the generative period of development is associated with the development of spore-bearing shoots, and an increase in the content of starch in the rhizome in the vegetative period was associated with the transition of plants to active photosynthesis and storage of its products in underground organs. The obtained microphotographs (see fig. 1a) illustrate the data on the high content of starch in the field horsetail rhizome at current stage of development.
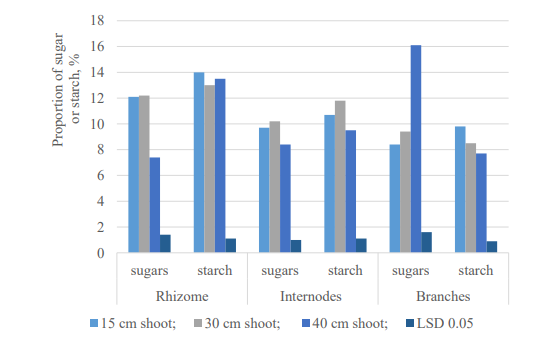
Fig. 2. Carbohydrate content of field horsetail (Equisetum arvense) in the vegetative period of development, % of air-dry weight
66
Structure and physiological state of field horsetail tissues
![]()
Since the leaves, as photosynthetic organs, are reduced in the assimilating shoots of horsetail, their function is performed by internodes and branches. Actively photosynthetic tissue (chlorenchyma) primarily underlies those parts of the epidermis of the shoots in which stomata are located. Previously, we found (Sytnikov et al., 2014) that almost the entire surface of the lateral ridges of the studied field horsetail branches is densely dotted with stomata, which are anatomically located above the chlorenchyma. Analysis of ultrastructure microphotographs of the cells of assimilating shoots of horsetail, taken with a transmission electron microscope, showed (see fig. 1 b, c) that the cells of the internode chlorenchyma were also in a functionally active state. Thus, here we have identified groups of photosynthetically active chloroplasts and mitochondria.
Chloroplasts here had the form of a thickened spindle 4–5 µm in diameter. The cell contained 5–15 chloroplasts; they contained a system of thylakoid membranes, a fine-grained stroma, and an electron-dense matrix. Their internal membrane system was located in the peripheral layers of the stroma, the main volume of chloroplasts was filled with starch grains of a simple structure, in the amount of 1–2 pieces (see fig. 1b).
Starch grains of chloroplasts are temporary stores of photosynthesis products. Chloroplasts of the upper, intensively growing internodes of young assimilating shoots (15 cm), as a rule, contain somewhat more starch grains per unit area than in the lower part of the plant. An analysis of the starch content in assimilating shoots (15 cm) of field horsetail (see fig. 2) also revealed a tendency for its increased accumulation (11.9 %) in the internodes of the upper part of the plant.
The study of sugars in assimilating shoots of field horsetail (see fig. 2) showed that their maximum amount is contained in the branches of plants (16.1 %). In the internodes, the amount of sugars was in the range of 8.4–10.2 % of the air-dry mass. The study of photosynthetic pigments showed that in the internodes of assimilating shoots, the average content of chlorophyll a was at the level of 0.60±0.03 mg/g and chlorophyll b – 0,25±0,01 mg/g, respectively. Previously, we showed (Sytnikov et al., 2014) that a relatively high content of photosynthetic pigments in the lower branches of assimilating shoots of field horsetail is due to the presence of a fully formed photosynthetic apparatus and indicates their high assimilative capacity. The data obtained are consistent with the results of studying the dynamics of the amount of sugars in the assimilating shoots of field horsetail and indicate the existence of a relationship between their content and photosynthesis processes provided by pigments.
CONCLUSIONS
The structural features of field horsetail rhizomes include poor development or complete absence of mechanical tissues and a large amount of storage parenchyma. In the phase of young assimilating shoots, the cells of the main rhizome parenchyma contain numerous amyloplasts, lipid drops, and mitochondria with a well-developed cristae system.
It is shown that in the generative period of development of field horsetail, the amount of starch in the rhizome is noticeably reduced, which is associated with the development of spore-bearing shoots. In the cells of the main parenchyma of the horsetail rhizome, in the phase of young assimilating shoots, there are many starch grains. The relatively high content of starch in the rhizome by the end of the vegetative period of development is due to the transition of plants to active photosynthesis with the subsequent storage of its products in these underground organs.
The highest sugar content was found in the rhizome in the generative period, as well as in the branches of assimilating shoots in the vegetative period of development. The relatively high content of sugars and photosynthetic pigments in the assimilating shoots of field horsetail is accompanied by the presence of groups of functionally active chloroplasts containing starch grains in chlorenchyma cells. This indicates the presence of the assimilation ability of a fully formed photosynthetic apparatus. The obtained data on the features of the ultrastructure of horsetail tissues in connection with the indicators of their physiological state can be used in studying the adaptive capacities of other representatives of pioneer vegetation.
67
Sytnikov D. M., Kucherik G. V.
![]()
References
Baydeman I. N. Methodology for studying the phenology of plants and plant communities. – Novosibirsk: Nauka, 1974. – 156 p. (in Russ.).
Botirov E. Kh., Bonacheva V. M., Kolomiets N. E. // Khimiya Rastitel’nogo Syr’ya. – 2021. – N 1. – P. 5–26. (In Russ.). DOI: 10.14258/jcprm.2021017760.
Dospekhov B. A. Field experiment methodology (with the basics of statistical processing of research results) – Мoscow: Agropromizdat, 1985. – 351 p. (in Russ.).
Flora UkrSSR / [Ed. O. V. Fomin]. – Kiev: Vydavnytstvo AN USRR, 1936. – Vol. 1. – P. 110–113. (in Ukr.).
Furst G. G. Methods of anatomical and histochemical study of plant tissues. – Moscow: Nauka, 1979. – 155 p. (in Russ.).
Gayer G. Electronic histochemistry. – Moscow: Mir, 1974. – 488 p. (in Russ.).
Kucheryava L. F., Voytyuk Yu. O., Nechitaylo V. A. Systematics of higher plants. I. Archegoniats. – Kiev:
Fitosotsiotsentr, 1997. – 136 p. (in Ukr.).
Marshall G. Growth and Development of Field Horsetail (Equisetum arvense L.) // Weed Science. – 1986. – Vol.
34. – P. 271–275. DOI: https://doi.org/10.1017/S0043174500066819.
Methods of biochemical research of plants / [Ed. A. I. Ermakov]. – Leningrad: Agropromizdat, 1989. – 430 p. (in Russ.).
Musienkо М. М., Parshykova T. V., Slavnyi P. S. Spectrophotometric methods in the practice of plant physiology, biochemistry and ecology. – Kiev: Fitosotsiotsentr, 2001. – 200 p. (in Ukr.).
Plant life [Ed.: Al. A. Fedorov], Moscow: Prosveshchenie, 1978. – Vol. 4. – 447 p. (In Russ.).
Shlyk A. A. Biochemical methods in plant physiology. – Мoscow: Nauka, 1975. – P. 154–170. (in Russ.). Sytnikov D. М., Babenko L. М, Shcherbatyuk М. М. Structure and physiological state of photosynthetic apparatus
of Equisetum arvense L. // Modern Phytomorphology. – 2014. – Vol. 5. – P. 215–219. DOI:
https://doi.org/10.5281/zenodo.161027
Сытников Д. М., Кучерик Г. В. Структура и физиологическое состояние тканей хвоща полевого // Экосистемы. 2022. Вып. 30. С. 64–68.
Изучена ультраструктура и физиологическое состояние основной паренхимы корневища, а также хлоренхимы вегетативных побегов хвоща полевого (Equisetum arvense L.). Для анатомического и ультраструктурного анализа тканей хвоща использовали сканирующий микроскоп JSM-6060 LA и трансмиссионный электронный микроскоп JEM-1230 (JEOL, Япония). Содержание растворимых и легкогидролизуемых углеводов определяли общепринятым методом, вычисляя массовую долю сахаров и крахмала
- процентах к воздушно-сухой массе. Содержание фотосинтетических пигментов определяли экстракцией хлорофилла 96 % этиловым спиртом с последующим определением оптической плотности экстрактов и расчетом концентрации пигментов в мг/г сырой массы. В клетках основной паренхимы корневища, содержащей максимальное количество крахмала, обнаружены амилопласты, заполненные зёрнами крахмала, липидные капли и митохондрии. Установлено наличие групп функционально активных хлоропластов в клетках хлоренхимы с высоким содержанием фотосинтетических пигментов и сахаров. Представленные данные о структуре и физиологических показателях состояния тканей дополняют представление об адаптационных возможностях хвоща полевого.
Ключевые слова: хвощ полевой, ультраструктура, физиологическое состояние тканей.
Поступила в редакцию 14.05.22
Принята к печати 23.06.22
68

