УДК 58.009:502.753
Pistacia atlantica (Anacardiaceae) in the South-Eastern Crimea: population characteristics, current status, protection
Letukhova V. Ju., Potapenko I. L.
Vyazemsky Karadag Scientific Station – Nature Reserve of RAS Feodosia, Russia
The article presents the results of monitoring of the population of a relict Mediterranean species Pistacia atlantica Desf listed in the Red Data Book of the Russian Federation. P. atlantica grows in the mountains of South Coast of the Crimea (from Balaklava to Karadag) in the coastal and lower mountain forest belts up to 400 m a.s.l., where it forms rare relict plant communities. Antropogenic impact on coastal landscapes is considered the main threat factor for P. atlantica communities. To date, the ancient Mediterranean forests have shrunk to small groves scattered along the coast, and therefore, they urgently need protection and monitoring. Active measures must be undertaken to restore particular plant species and population. The field research was conducted in 2018–2020. The researchers identified and studied seven populations, four of which had natural origin and three had artificial origin. Sample plots were set up at each site to count the total number of P. atlantica plants and determine their age states. Biomorphological indicators of generative plants were also measured: tree height, number of trunks, trunk diameters at the at the ground level and at the height of 1.3 m. Ontogenetic structure of P. atlantica populations was studied at each site. The age index (Δ), the mean efficiency index (ω) and the regeneration index were calculated and the type of the populations was determined (according to the “delta–omega” (∆–ω) classification). Native populations were identified as young ones (age index (Δ) 0.17–0.24, mean efficiency index (ω) 0.45–0.56 and regeneration index 43.2–158.7 %). They had the left-sided spectra: the pregenerative individuals dominated there. The artificial populations were maturing or mature: age index (Δ) 0.30–0.51, recovery efficiency index (ω) 0.70–0.76, regeneration index 12.8–20.5 %). They had either centered (young or middle-aged generative individuals dominated) or right-sided (old generative individuals dominated) spectra. The average height of the trees in the populations varied from 3.0 to 5.5 m. The authors found a significant positive correlation between this parameter and annual precipitation values. The average number of tree trunks in the populations ranged from 1.4 to 2.4. This parameter is significantly and negatively correlated with annual amount of precipitation. On the other hand, the temperature effect on the morphological parameters was statistically insignificant. The state of all the studied populations was considered normal, general phytosanitary state of the trees was satisfactory. A new locality of P. atlantica was found, thus, it was proved that the habitat of this species expanded to Feodosiya. The main risk factor for this species in the South-Eastern Crimea is the habitats reduction due to anthropogenic pressure on natural areas and resort construction.
Key words: impact of climate, Crimean Peninsula, monitoring, ontogenetic structure, rare species, Pistacia atlantica.
INTRODUCTION
The genus Pistacia belonging to Anacardiaceae family includes the evergreen, half-evergreen and deciduous trees and shrubs with pari- and imparipinnate leaves (El Zerey-Belaskri, 2019). For a long time Pistacia atlantica Desf was known in Russia as P. mutica Fisсh. & C. A. Mey. (Czerepanov, 1995). P. mutica was described by Fischer and Meyer (1838), while P. atlantica was classified by Desfontaines (1799). The only characteristics by which the first species differs from the second one are the ovate form of the leaflets and their lower number. Zohary (1952) who made the first classification of the genus, had no decision whether P. mutica was a separate species and considered it as P. atlantica subsp. mutica. The current studies showed that there was no genetic distance between P. atlanthica and P. mutica and they should be considered within the same species (Kafkas, 2006; Al-Saghir, 2010).
Pistacia atlantica is a relict Mediterranean species, listed in the Red Data Book of the Russian Federation (Litvinskaya, 2008) as P. mutica with category 3 (rare species). It is distributed in the Near East, West and Central Asia, North Africa, the Balkan Peninsula, the Caucasus and in the Crimea. In the Crimea, P. atlantica grows in the South Coast of the Crimean Mountains (from Balaklava to Karadag) in the coastal and lower mountain forest belts up to 400 m a.s.l., and some fragments are in the western part of the foothills (Sevastopol–Bakhchysarai). This species forms rare
ISSN 2414-4738 Published by V. I. Vernadsky Crimean Federal University, Simferopol
relict communities (Green Data Book of the Ukraine, 2009). The communities are scares sparse hemixerophytic woodlands on dry rubbly slopes with brown soils of two-three-layer structure where
P. atlantica is a dominant and edificator. It also inhabits juniper (Juniperus excelsa M. Bieb.) and oak (Quercus pubescens Willd.) forests (Jasmino—Juniperion excelsae alliance, Quercetea pubescentis—petraea class). The number of P. atlantica individuals is high. It grows as single trees, in small groups, or in communities over vast areas. Threat factors for the species in the Crimea are anthropogenic impact on coastal landscapes and weak competitive ability of the species (Letukhova et al., 2016; Shilovskaya, Goncharenko, 2016). Thus, the study of P. atlantica in its native habitats is of current interest.
Monitoring of rare species is a system of regular observations, assessment and forecast of their state in nature. This is one of the most important areas of biodiversity monitoring in the world. Its goal is to identify negative changes, as well as to prevent and eliminate these changes (Bychenko, 2008; Matveenko, Rubtzova, 2009; Mccall, 2017). Monitoring is a powerful tool for identifying problems in the early stages, before they become dramatically obvious or crises. If identified early, problems can be addressed while cost-effective solutions are still available (Carpenter et al., 1999). The idea of the state of a species with a more or less wide range is formed on the information about the state of its individual populations. Monitoring of the state of P. atlantica is carried out throughout its range in the Crimea: from Karadag to Sevastopol (Shevchenko & Vasilieva, 1992; Shilovskaya & Goncharenko, 2016; Chernyshova et al., 2018; Rebriev & Sokolova, 2020). The studies were carried out mainly using forest inventory methods (Shilovskaya, 2018; Yarysh et al., 2019; Yarysh & Yarysh, 2020). However, the population-ontogenetic method, widely used in modern biology, allows us to study the state of native populations in various ecological and phytocenotic conditions and predict their further development under the influence of biotic and abiotic factors (Osmanova & Zhivotovsky, 2020). A comprehensive work was carried out in the Besh-Tash valley in 2013 (Letukhova et al., 2016). The state of the population was satisfactory. Trees of all age stages were found (without senile), young individuals predominated. Most of the trees (73.8 %) were single- stemmed, which indicates favorable conditions and low anthropogenic impact.
This paper was aimed at studying Pistacia atlantica population status in the South-Eastern Crimea. The following tasks were set: to inventory the localities of the species and determine the current boundaries range, study the state and age structure of populations, identify factors that affect the growth and development of the species.
MATERIAL AND METHODS
The investigated area covers the southern slopes of the Main ridge of the Crimean Mountains from Alushta in the west to Feodosia in the east (about 90 km). Vegetation of the South-Eastern Coast is a belt (to a height of 350–400 m a.s.l.) of xerophytic oak-juniper (Quercus pubescens– Juniperus excelsa), oak-terebinth (Quercus pubescens–Pistacia atlantica), oak-eastern hornbeam (Quercus pubescens–Carpinus orientalis) woodlands. At some places there are shrublands dominated by shrubby forms of Quercus pubescens, P. atlantica, Carpinus orientalis Mill., and species such as Juniperus deltoides R. P. Adams, Crataegus orientalis Pall. ex Bieb., Cotoneaster tauricus Pojark., Rosa corymbifera Borkh., Rhus coriaria L., Cotinus coggigria Scop., Paliurus spina-christi Mill., among others. The Mediterranean elements of the flora are fading from west to east, while more xerophytic steppes prevailin the east. The climate of the study area has its specific features due to the combination of steppes, mountain ranges, foothills and the warm sea. It changes from sub-Mediterranean in the west to temperate continental in the east. The temperature regime differs slightly throughout the study region (tabl. 1). The average annual air temperature ranges from
+11.7 °C to +12.3 °C. Cape Meganom is the driest area (annual precipitation amount is 272 mm). The amount of precipitation increases to the west (towards Alushta) and to the east (towards Feodosia). As a whole, the region Alushta–Sudak–Feodosia is characterized by dry air and low rainfall level.
Table 1
Climatic characteristics of the South-Eastern Crimea, Eastern Europe (according to Bagrova et al., 2001)
| A location of meteostation | Average air temperatures, °C | Precipitation, mm | ||||
| January | July | Annual | November –
March |
April –
October |
Annual | |
| Alushta | +3.0 | +23.3 | +12.3 | 225 | 202 | 427 |
| Sudak | +1.8 | +23.2 | +11.9 | 129 | 189 | 318 |
| Meganom
lighthouse |
+1.6 | +23.8 | +12.0 | 115 | 157 | 272 |
| Karadag | +1.5 | +23.4 | +12.1 | 146 | 211 | 357 |
| Feodosia | –0.6 | +23.8 | +11.7 | 151 | 225 | 376 |

Fig. 1. Pistacia atlantica in the South-Eastern Crimea
A – Trees growing on the territory of increased recreational load (Pop4). B – Trees growing in artificial planting by the rows (Pop5). C – Trees growing on loose slopes of sea coast (Karadag). D – The tree in autumn foliage colour.
The field studies were conducted in 2018–2020 in the South-Eastern Crimea using population- based and geobotanical methods (Rabotnov, 1992; Mirkin et al., 2001; Zhivotovsky, 2001). We examined seven P. atlantica populations (fig. 1–2). In each of them a study site was identified where plant individuals were counted, taking into account their age states.
The age states of trees were given according to the characteristics described earlier (Letukhova et al., 2016) with some clarifications. An immature plant (im) has height of 0.2–2.0 m; stem diameter at the ground level is 0.5–3.5 cm. In this age state, a monopodial shoot system with second order branches develops. Though a definite crown is not formed, mature leaves are present (no juvenile leaves). Virginile plant (v) has height 2.0–6.0 m; stem diameter at the ground level is 1.5–7.5 cm. It
has a typical tree-form, with a clearly-defined tree stem surmounted by a brunched crown; neither flower nor bear fruit. A young generative plant (g1) has height 1.5–10.0 m; stem diameter at the ground level is 4.5–10.5 cm; stem diameter at a height of 1.3 m is 2.0–9.5 cm. A top of a tree crown is acute; show maximum annual leader-shoot growth; flowering and fruiting begin but fruiting is irregular, not abundant, and usually in the upper part of the crown. A mature generative plant (g2) has height 2.0–10.0 m; stem diameter at the ground level is 11.0–30.0 cm; stem diameter at a height of 1.3 m is 3.0–25.0 cm. It has a crown with maximum width in the middle or upper part; the tree stem is covered with thick bark with small cracks in it. Several processes indicate ageing: reduced annual shoot elongation, delayed leader-shoot elongation. As a result, trees have well developed obtuse crown. Flowering and fruiting are regular and abundant. An old generative plant (g3) has height 2.5–10.0 m; stem diameter at the ground level is 31.0–62.0 cm; stem diameter at a height of
1.3 m is 6.0–32.0 cm. It has a wide-round crown as height growth has virtually stopped. Dying of large branches and top of the crown begins. The stem bark is deeply fissured as a result of dead tissue accumulation. The stem growth continues in thickness, but annual shoot elongation diminishes sharply. Fruiting ability diminishes. Senile plant (s) has dead tree top, many dying large branches, so a more open crown is present with maximum width in its upper part. There is no fruiting.
To describe a plant community with P. atlantica, the methods of geobotanical studies were used; for this, the complete species composition of plant community in the study plot, as well as its vertical and horizontal structure, was identified. Species names were provided according to «POWO» (http://www.plantsoftheworldonline.org).
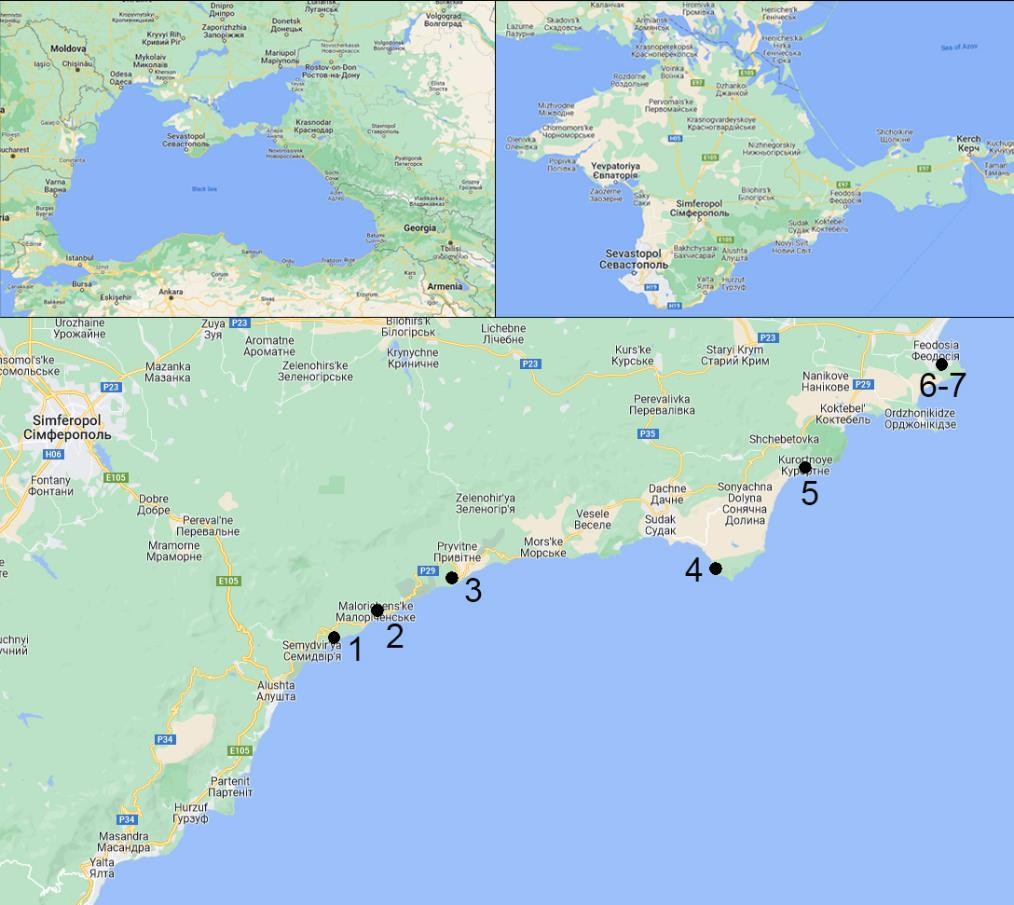
Fig. 2. The map of Pistacia atlantica populations studied in the South-Eastern Crimea (the numbers represent the population numbers) (background maps were taken from © Google 2022).
To assess the age level, the age index (Δ) (according to Uranov, 1975; Uranov, Serebryakova, 1976; Serebryakova, 1977; with modifications of Osmanova & Zhivotovsky, 2020) and the mean efficiency index (ω) (according to Zhivotovsky, 2001) were applied. The population type was described according to the “delta–omega” (∆–ω) classification (Zhivotovsky, 2001). To assess the
regeneration, we used the regeneration index (IB) (Kricsfalusy, Mező-Kricsfalusy, 1994), calculated as the percentage of the number of plantlets, juvenile, and immature plants to the number of generative individuals. Biomorphological parameters of plant individuals were measured at the study plots: height of a tree, number of tree stems, stem diameters at the ground level and at the height of
1.3 m. The trees with one stem were identified as single-stemmed; with two and three stems were identified as few-stemmed; with more than three stems were done as multi-stemmed.
Preliminary processing and analysis of data were conducted using Microsoft Office Excel 2010. Statistical analysis was performed in R version 4.1.0 (R Core Team, 2021). The Shapiro Wilk W- test rejected the observations’ normality of distribution. W varied from 0.863 to 0.956 that were not within the 95 % critical value accepted range. So we used non-parametric methods for statistical analysis (Yantzev, 2007). The Mann–Whitney U-test was used to explore if there were significant differences in the morphological parameters between different populations. Spearman’s rank correlations were calculated between climatic characteristics of the region and mean values of morphological parameters in the population. The Chaddock scale was used for the assessment of the significance of the correlation between two variables. Morphological parameters within Pop7 were measured but not analyzed due to a statistically small sample size (only two generative plant individuals were noted there).
RESULTS
Pop1 is situated in Sotera on the territory of the regional Protected Area “Pistacia atlantica woods” with 0.043 km2 (formed in 1997). It was established specially for the complex preservation of the plant community with P. atlantica. The study plot is situated between Alushta and Solnechnogorskoye (15 km to the east of Alushta and 10 km to the west of Solnechnogorskoye).Vegetation is the oak-terebinth (Quercus pubescens–Pistacia atlantica) woodland. In the plant community P. atlantica co-exists with trees Quercus pubescens, Juniperus excelsa, Carpinus orientalis; shrubs Paliurus spina-christi, Pyrus elaeagrifolia Pall.; herbs Festuca valesiaca Schleich. ex Gaudin, Dianthus capitatus subsp. capitatus, Teucrium chamaedrys L.,
T. polium L., Artemisia taurica Willd.
Pop2 is situated half way between settlements Rybachye and Malorechenskoe to the right of the highway Sudak–Alushta. There is an artificial planting of P. atlantica with the area of approximately
0.025 km2. It is the homogeneous thickets of rows of the terebinth trees. There are no shrubs. The herb layer is the following: Festuca valesiaca, Taraxacum serotinum (Waldst. et Kit.) Poir., Teucrium polium, Artemisia taurica, Asparagus verticillatus L.
Pop3 is situated 3 km from settlement Pryvetnoye towards Rybachye to the left of the highway Sudak–Alushta on the ascent to the Staurunyn-Burun mount. Vegetation is the oak-terebinth (Quercus pubescens–Pistacia atlantica) woodland where P. atlantica is a dominant. The shrub layer is poorly developed (Paliurus spina-christi, Rosa sp.). The herb layer is the following: Festuca valesiaca, Artemisia taurica, Galatella villosa (L.) Rchb. f., Salvia nemorosa subsp. pseudosylvestris (Stapf) Bornm., Eryngium campestre L., Teucrium polium. Terebinth trees grow irregularly there: rather thick in gullies (the canopy density is about 80 %) and single trees on the slopes. We saw signs of a fire (burnt stones and tree stems) that probably happened a few years ago. The vegetation is in the late stages of pyrogenic succession.
Pop4 is situated on the Rybachy cape 8 km east towards Sudak. It is the territory of the State Nature Monument “Meganom peninsula” with 6.52 km2 (formed in 2007) for the protection and preservation of landscape and biotope diversity of this area. Vegetation is the terebinth woodland, where P. atlantica co-exists with shrubs Pyrus elaeagrifolia; herbs Festuca valesiaca, Galatella villosa, Eryngium campestre. The herb layer is heavily degraded in some places because of the recreation load (many paths, campsites and campfires).
Pop5 is situated on Karadag plateau near Karadag Scientific Station. It is the territory of the State Nature Reserve “Karadagsky” with 28 km2 (formed in 1979). It has a conservation status of the
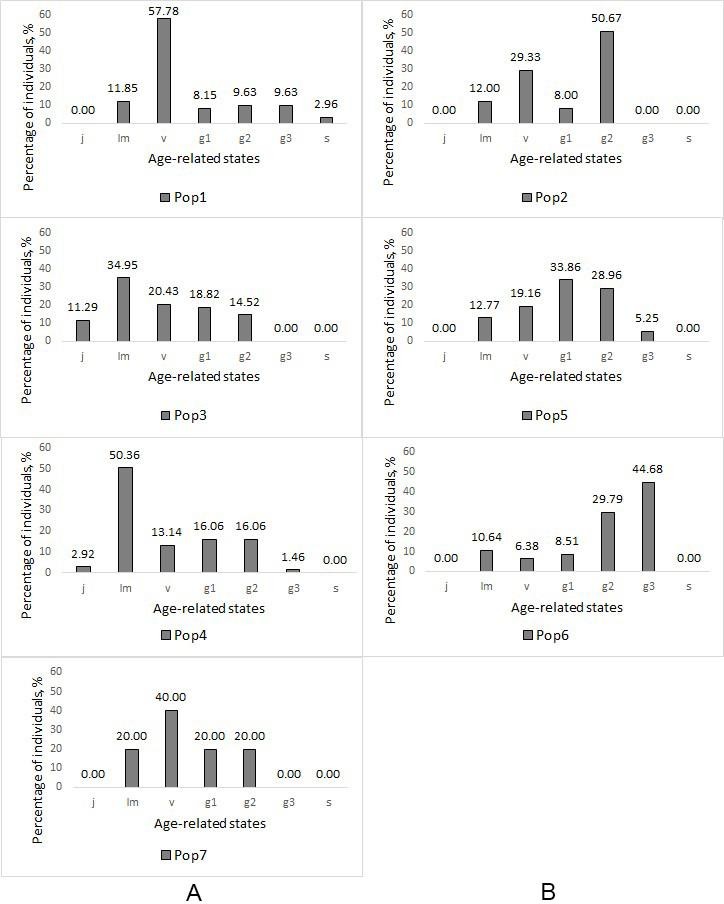
Fig. 3. Ontogenetic spectra of the Pistacia atlantica populations at the study plots
Note: A – populations of native origin; B – populations of artificial origin. j – juvenile plants; im – immature plants; v – virginile plants; g1 – young generative plants; g2 – mature generative plants; g3 – old generative plants; s – senile plants.
highest priority category for biodiversity conservation in Crimea (Biodiversity Support Program, 1999). The reserve is situated between settlements Kurortnoye and Koktebel. It is an artificial planting of P. atlantica (fig. 3B). Its total area is 25 000 m2. The planting was made approximately in 1960–1970s. In some places there are homogeneous thickets of tree rows; in other places the trees are rare. At the shrub layer there are single trees of Pyrus elaeagrifolia; the herb layer is the following: Festuca valesiaca, Galatella villosa, Stipa pontica P. Smirn., Artemisia austriaca Jacq., as well as invasive species Opuntia humifusa (Raf.) Raf., O. phaeacantha Engelm.
Pop6 and Pop7 are situated on the territory of the State Nature Sanctuary “Mountain Group Tepe-Oba” with 12 km2 (formed in 2007). The Tepe-Oba mountain range located near the city of Feodosia stretches 8–10 km from east to west and has a maximum altitude of 290 m a.s.l. Vegetation is a degraded deciduous forest (dominated by Quercus pubescens and Carpinus orientalis). A considerable part of the northern slope and the tops of the ridge are occupied by artificial planting of pine-trees (Pinus nigra subsp. pallasiana (Lamb.). Holmboe) and sometimes deciduous trees and shrubs (Acer tataricum L., Fraxinus excelsior subsp. excelsior, Juglans regia L., Prunus mahaleb L., Laburnum anagyroides Medik., Rhus coriaria). The plantings were carried out throughout the 20th century, but especially intensively in 1970s in order to recreate the destroyed forests. At present they are heavily degraded. We found P. atlantica in these plantings, which have not been noted before (Shatko, Mironova, 2011).
Pop6 is situated in the artificial planting of P. atlantica. Terebinth trees grow very densely and co-exists with the trees: Fraxinus excelsior subsp. excelsior, Pinus nigra subsp. pallasiana, Juniperus excelsa, Prunus mahaleb. The shrub layer is well-developed (Laburnum anagyroides, Ligustrum vulgare L., Crataegus rhipidophylla var. rhipidophylla, Cotoneaster tauricus). The herb layer is poorly developed due to a hard forest litter. There are Galatella villosa, Elymus repens (L.) Gould, Festuca valesiaca there.
Pop7 is situated at the site where P. atlantica expanded its growth area, about 100 m from the artificial planting. It is small in number and area. But this population is interesting as one which is dispersal off the natural range. In the plant community P. atlantica co-exists with trees as Fraxinus excelsior subsp. excelsior, Prunus amygdalus Batsch, Juniperus excelsa; shrubs as Ligustrum vulgare, Cornus mas L., Laburnum anagyroides, Crataegus rhipidophylla Gand.; herbs as Dactylis glomerata L., Stipa pennata L., Festuca valesiaca, Galatella villosa, Artemisia austriaca, Teucrium chamaedrys, Eryngium campestre, Achillea setacea Waldst.&Kit. More detailed characteristics of the study plots are given in table 2.
Thus, we observed seven P. atlantica populations in the South-Eastern Crimea, four of them are of native origin, and three are artificial. Five populations occupy Protected Areas; two are the territories, which don’t have any conservation status. There were the visible signs of negative human impact on some of them.
The highest total density was within Pop2 – 1250 individuals per 10 000 m2 (generative – 733 individuals per 10 000 m2). It was because of the thickness of trees planting there (tabl. 3). Pop7 has the lowest total density – 20 individuals per 10 000 m2 (generative – 8 individuals per 10 000 m2). It was because the population began its development not long ago. Pop6 has the highest (0.51) age index and the lowest (12.8 %) regeneration index. Pop3 and Pop4 have the highest regeneration indexes – 141 % and 158.9 % correspondently. We identified three types of populations according to “delta-omega” classification: all the native populations were young, two artificial populations (Pop2 and Pop5) were maturing, and one (Pop6) was mature.
All studied populations were normal (self-sustaining, not dependening on the seeds introduction from outside), but incomplete (do not have individuals of one or several age states) (fig. 3). They often had not senile (s) plants (Pop2, Pop3, Pop4, Pop5, Pop6, Pop7), juvenile (j) plants (Pop1, Pop2, Pop5, Pop6, Pop7), and old generative (g3) plants (Pop2, Pop3, Pop7). We identified three types of ontogenetic spectra in our sample plots depending on their origin. Populations of native origin were characterized by a left-sided spectrum with a maximum on the pregenerative part: immature (im) individuals (Pop3 and Pop4) or virginal (v) individuals (Pop1 and Pop7). In artificial populations, either a centered spectrum was observed with maxima on young generative (g1) individuals (Pop5) and middle-aged generative (g2) individuals (Pop2) or a right-hand spectrum with a maximum on old generative (g3) individuals (Pop6).
Morphological parameters of generative plant individuals were as follows: the largest mean diameters at the ground level and at a height of 1.3 m were noted in Pop6 (correspondently 24.5 cm and 16.0 cm), the lowest values of these parameters were in Pop3 (correspondently 10.6 cm and
4.9 cm). The number of tree stem in the populations varied from 1 to 11. Based on the Mann–Whitney U-test, we divided all populations into two groups. The first group included the populations with
mostly single- and few-stemmed trees (Pop1, Pop3, Pop6). The second one included the populations with mostly few- and multi-stemmed trees (Pop2, Pop4). The multi-stemness differences between populations within every group were not statistically significant (P=0.182–0.304). On the other hand, the difference in this parameter between populations from different groups was statistically significant (P<0.05).
Table 2
Characteristics of the study plots with Pistacia atlantica in the South-Eastern Crimea
| Study plot | Locality / coordinates | Plant association | Altitu- de, m a.s.l. | Aspect / Steepness, degrees | Tree layer | Shrub layer | Herb layer | |||
| Forest stands | Canopy density,
% |
Height, m | Cove- rage, % | Height, m | Cove- rage, % | |||||
| Pop1 | Sotera / 44°43’47.4″ N 34°29’03.0″
E |
Querceto- Pistacietum festuceto- teucriumosum | 155 | NW / 7 | T4+O4+ Ju1+H1 | 60 | 4 | 10 | 2 | 50 |
| Pop2 | Between Rybachye and Malore- chenskoe /
44°45’41.3″ N 34°34’58.6″ E |
Pistacietum festucosum | 90 | SSW / 10 | T9+O1 | 70 | 3 | 10 | 1.5 | 10 |
| Pop3 | Near Pryvetnoye
/ 44°47’55.3″ N 34°40’35.0″ E |
Pistacietum festucosum | 90 | NW / 20 | T8O2 | 60 | 3,5 | 10 | 1.5 | 60 |
| Pop4 | Rybachy cape / 44°48’27.4″ N 35°02’60.0″
E |
Pistacietum festucosum | 10 | W / 5 | T10 | 40 | 2.5 | 10 | 1 | 40 |
| Pop5 | Kara-Dag / 44°54’54.9″ N
35°12’18.5″ E |
Pistacietum festuceto- gallatelo villosum | 10 | 0 | T10+Psp | 50 | 5 | 5 | 1.2 | 60 |
| Pop6 | Tepe-Oba / 45°00’32.4″ N 35°23’28.1″
E |
Pistacietum ligustroso gallatelo villosum | 164 | N / 5 | T9+A1+
PnspChsp |
80 | 7 | 60 | 1.6 | 30 |
| Pop7 | Tepe-Oba / 45°00’28.5″ N 35°23’26.1″
E |
Fraxinetum ligustroso dactylo glomeratum | 164 | N / 5 | A5+T2+ Al1+
Jusp |
60 | 5 | 30 | 1.3 | 70 |
Note. Forest stands: T – Pistacia atlantica; O – Quercus pubescens; Ju – Juniperus excelsa; H – Carpinus orientalis; P – Pyrus elaeagrifolia; A – Fraxinus excelsior subsp. excelsior; Pn – Pinus nigra subsp. pallasiana; Ch – Prunus mahaleb; Al – Prunus amygdalus; sp – sporadically.
Table 3
Characteristics of the Pistacia atlantica populations at the study plots
| Study plot | Parameters of the populations | Population’s types | |||||
| Density of individuals per 10 000 m2 | Age index, Δ | Efficiency index, ω | Regeneration index, % | ||||
| 1 | 2 | 3 | |||||
| Pop1 | 376 | 164 | 540 | 0.24 | 0.51 | 43.2 | young |
| Pop2 | 517 | 733 | 1250 | 0.31 | 0.71 | 20.5 | maturing |
| Pop3 | 496 | 244 | 740 | 0.17 | 0.45 | 141.0 | young |
| Pop4 | 364 | 184 | 548 | 0.17 | 0.46 | 158.7 | young |
| Pop5 | 146 | 311 | 457 | 0.30 | 0.70 | 18.8 | maturing |
| Pop6 | 32 | 156 | 188 | 0.51 | 0.76 | 12.8 | mature |
| Pop7 | 12 | 8 | 20 | 0.21 | 0.56 | 50.0 | young |
Note. 1 – density of pregeneretive plant individuals; 2 – density of generetive plant individuals; 3 – total density of the population.
Pop4 (tabl. 4) had the lowest average tree height (3.0 m). Average tree height increased to the west towards Alushta and to the east towards Feodosia: it was 3.9 m in Pop1 and 5.5 m in Pop6. According to Mann Whitney U-test, the difference between the values of the trees height in Pop4 (where these values were the smallest) and all other populations was statistically significant (P<0.01).
Table 4
Mean values of morphological parameters of Pistacia atlantica generative individuals in the study plots
| Study plot | Morphological parameter | |||||||
| Height, m | Number of tree
stems |
Stem diameter at the
ground level, cm |
Stem diameter at a
height of 1.3 m, cm |
|||||
| M±m | max– min | M±m | max– min | M±m | max–min | M±m | max– min | |
| Pop1 | 3.9±0.4 | 6.0–1.5 | 1.4±0.2 | 3–1 | 20.2±3.3 | 45.0–5.0 | 12.5±2.7 | 30.0–2.0 |
| Pop2 | 3.8±0.3 | 6.0–2.0 | 2.3±0.4 | 6–1 | 15.1±1.5 | 25.0–5.0 | 8.7±1.2 | 15.0–4.0 |
| Pop3 | 3.6±0.2 | 5.5–1.8 | 1.8±0.3 | 7–1 | 10.6±1.4 | 25.0–4.0 | 4.9±0.8 | 16.0–1.5 |
| Pop4 | 3.0±0.4 | 6.0–1.0 | 2.4±0.6 | 11–1 | 12.6±2.0 | 32.0–3.0 | 8.2±1.8 | 16.0–3.0 |
| Pop5 | 3.7±0.1 | 6.5–1.5 | – | – | 14.9±0.6 | 60.0–4.5 | 8.7±0.4 | 32.0–2.0 |
| Pop6 | 5.5±0.4 | 7.0–3.0 | 1.4±0.2 | 5–1 | 24.5±4.4 | 50.0–5.0 | 16.0±3.9 | 38.0–1.0 |
We analysed the correlation between the mean values of the morphological parameters of the populations and environmental factors (tabl. 5). We did not analyse parameters of Pop2 because we didn’t have climatic characteristics for this study plot. We found a significant positive correlation between mean tree height and annual precipitation amount (r=0.90; P=0.037), whereas the mean number of tree stems was significantly and negatively correlated with this climatic parameter (r=–0.95; P=0.05). The effect of a temperature on the mean value of trees morphological parameters in populations was statistically nonsignificant.
Table 5
Spearman’s rank correlations between climatic characteristics and morphological parameters in
Pistacia atlantica populations in the South-Eastern Crimea
| Climatic characteristics | Mean values of morphological parameters | |||||
| Height | Number of tree
stems |
Stem diameter at the
ground level |
||||
| A | B | A | B | A | B | |
| Average long-term air | r=–0.10; | weak | r=0.10; | weak | r=0; P=1 | non |
| temperatures | P=0.873 | P=0.895 | ||||
| Average temperatures of the coldest month
(January) |
r=–0.30; P=0.624 | weak | r=–0.10; P=0.895 | weak | r=–0.40; P=0.505 | moderate |
| Average temperatures of | r=0.10; | weak | r=0.10; | weak | r=0.50; | moderate |
| the warmest month (July) | P=0.870 | P=0.944 | P=0.434 | |||
| Annual precipitation | r=0.90; | high | r=–0.95; | very | r=0.80; | high |
| amount | P=0.037 | P=0.051 | high | P=0.104 | ||
Note. A – Spearman’s rank correlation coefficient (r) and P-value; B – qualitative measure of the coefficient on Chaddock’s scale. Parameters in which we found a statistically significant correlation (with P ≤ 0.05) are in bold.
DISCUSSION
At present there is an acute issue of preserving endangered species and biodiversity in general. Population investigations of rare species are the part of long-term monitoring and the basis of nature conservation activity. They include not only visual assessments but also take into account different parameters that characterize the development of plants in a certain plant community. The effectiveness of protection and recovery depends on proper understanding of populations’ onthogenetic and morphological characteristics and their dynamics (Glazkova, 2021; Isayeva, 2022). Pistacia atlantica is a local species scattered in most of its localities. The abundance of the species varies from rare, occasional to abundant in few stations but most of the subpopulations are found as isolated trees or as small clusters of 2–3 trees (IUCN, 2022). In Morocco, P. atlantica occupies large areas in the eastern region, but in scattered and isolated state. It has the smallest density of population here. The density varies from 20 individuals over an area of 8000 m2, 80 to 100 individuals over 1 km2 and 30 to 50 individuals over 1 km2 (Faouzi et al., 2015). In Algeria, P. atlantica exists in small stands or as isolated individual trees scattered often outside forests (Benradje et al., 2012). In the South-Earstern Crimea P. athlantica occupies not large area, but it has high
density of population (it varies from 2000 to 125 000 over 1 km2).
A state of all the studied populations is normal: they have all age states individuals. General phytosanitary state of the trees is satisfactory. Artificial populations are dominated by generative individuals, the age index is high. The seedlings were planted closer together. It prevents at present the renewal and rejuvenation of P. atlantica. They are considered as maturing and mature and need further monitoring.
The lowest values of the age indices and the highest values of the renewal indices are at sites with anthropogenic pressure (Pop3 and Pop4). The permanent (Pop4) or temporary (Pop3) disturbance of the herb cover would be the reason: a dense network of paths, traces of fire, and trampling of herbaceous vegetation (fig. 3A). The closed herbaceous layer forms a dense turf, preventing the penetration into the ground and the germination of tree seeds. The disturbance of the turf improves the conditions for the seed renewal. According to Koba & Zhigalova (2014) detritus in forests, formed after fires, reduces the germination energy and seed germination of herbaceous plants and slows down the formation of the herbaceous layer. At the same time, the seeds of woody
plants (in particular, Pinus nigra subsp. pallasiana) germinated quite successfully on various types of detritus.
According to Pulliam (1988), habitat patches supporting population sources can produce a surplus of individuals to disperse to adjacent habitats. We observed this phenomenon at Tepe-Oba, where P. atlantica spread from the artificial plantings to natural habitats. We discovered a new locality near the artificial planting. It was the small population, but its individual parameters (ontogenetic spectrum, indexes of age (Δ), efficiency (ω) and renewal) indicated stability and normal state. Artificial populations can serve as a genetic reserve and play an important role in expanding the range and spreading the species to new territories (Shilovskaya, Goncharenko, 2016).
Studies of the influence of climatic conditions on the morphological parameters of tree species were carried out earlier. It was found that the overall predominance factor in determining tree height growth was temperature, which positively correlated with a tree height (Kesslera et al., 2007; Messaoud & Chen, 2011; Lines et al., 2012; Zhou et al., 2019). In some species (Quercus ilex L., Torminalis glaberrima (Gand.) Sennikov & Kurtto) there was for a greater effect of precipitation on tree height compared to the effect of temperature (Fortin et al., 2019). Ontomorphogenesis and the formation of life forms in various species of woody plants were well studied by other researchers (Chistyakova, 1988; Bellingham & Sparrow, 2009; Tanentzap et al., 2012). The formation of multi- stemmness may be due to environmental conditions (Nedoseko, 2012), climate (Mazepa & Devi, 2007), exogenous factor (damage to the main stem by wildfire) (Yasinskaya et al., 2018). Morphological parameters of trees of the studied populations weakly correlated with the temperature. We suppose, that the temperature variability is not sufficient here. The tree height significantly and positively correlated with precipitations. The multi-stemness significantly and negatively correlated with precipitations. The population studies have shown that precipitations affect P. atlantica individuals height and number of stems more considerably than temperature in arid climate of South- Eastern Crimea. The fact that climatic factors affect height growth more than diameter growth are in accordance with other authors (Way & Oren, 2010).
P. atlantica is a rare protected species needing substantial attention. It often grows at areas which are very attractive for resort construction and its growth is often reduced due to the human impact. Therefore, the most effective measures for the conservation of this species are still the creation of new and the expansion of previously created protected areas, where economic activity will be limited. The other important method of conservation is its cultivation in botanical gardens and forestries where you can get seed and planting material for reintroduction to natural habitats.
The species is able to tolerate most soil conditions including alcaline and will survive with no irrigation. It also withstands heat and winds (Rezaeyan et al., 2009). It is important its value against soil erosion. It strengthens the soil, is used for afforestation of arid and steep slopes and against landslides (Ginsberg, 2000; Amara et al., 2017; Hosseini et al., 2021). P. atlantica is able to grow on loose slopes of sea coasts, are tolerant to sea winds, splashes and aerosols (fig. 3C). It can be used in dryland reforestation against erosion. P. atlantica is planted as an ornamental shadebearing and drought-tolerant landscape tree in gardens and parks. It is an ornamental deciduous tree growing up to 7−10 m tall with spreading branches and forms a dense crown. The terebinth tree is especially decorative during fruit ripening and autumn foliage color (fig. 3D). The oblong, fleshy, oily fruit borne by the female tree is 6 to 8 mm long and pink in color, ripening to blue. The spreading crown allows it to be used as a shade tree in gardens and parks. Cultivating them in gardens and parks will serve as an additional measure to preserve the species (Potapenko, 2010; Potapenko et al., 2021). Countries in the Mediterranean and Middle Eastern areas cultivate P. atlantica for economic, medical and other applications. Various industrial and traditional uses are mentioned for the main parts of
P. atlantica (resin and fruit) including using for food and medicine. Recent research investigates the wide pharmacological properties of P. atlantica’s various parts, such as antimicrobial, antioxidant, antidiabetic, antitumor, and antihyperlipidemic (Gourine et al., 2010; Mahjoub et al., 2018; Labdelli et al., 2019). Such applications of P. atlantica can be implemented in our country in the future.
P. atlantica has remarkable morphological variability in its large geographical distribution area. Therefore, for avoiding any genetic pollution in the case of in-situ and ex-situ conservation programs
El Zerey-Belaskri (2019) recommended the following: seeds from every population or at least from every area should be used to produce seedlings for the restoration of the same population. On the other hand, phenotypic variability is observed even in such a relatively small area as the South- Eastern Crimea. For instance, we found there trees with larger leaves than in other locations. We recommend to take seeds from such trees to grow more decorative seedlings and plant them in parks and green areas of settlements and recreational areas of the Crimea.
CONCLUSIONS
P. atlantica had satisfactory state throughout its habitats in the South-East Crimea. All populations were normal, but incomplete due to the absence of one or another age state in their age spectra. Native populations had a left-sided spectrum, while artificial populations had a centered or right-sided spectrum. Native populations were young, artificial were maturing and mature. The expansion of habitats to the east was revealed, new location of P. atlantica was foundon the Tepe- Oba near Feodosia. Climate affects the morphological characteristics of P. atlantica. Mean height of the trees and number of tree stem were positively correlated with annual precipitation. On the other hand, the effect of temperature on the morphological parameters was statistically nonsignificant in the conditions of the South-Eastern Crimea.
The work was supported by the research project of T.I. Vyazemsky Karadag Scientific Station – Nature Reserve of RAS – Branch of A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS (121032300023-7).
References
Al-Saghir M. G. Phylogenetic analysis of the genus Pistacia L. (Anacardiaceae) based on morphological data // Asian Journal of Plant Sciences. – 2010. – Vol. 9. – P. 28–35.
Amara M., Bouazza M., Al-Saghir M. G. Anatomical and adaptation features of Pistacia atlantica Desf. to adverse climate conditions in Algeria // American Journal of Plant Sciences. – 2017. – Vol. 8, N 2. – P. 137–153.
Bagrova L. A., Bokov V. A., Bagrov N. V. Geography of the Crimea: manual for educational institution’s students. – Kiev: Lybid, 2001. – 304 p. [In Russian]
Bellingham P. J., Sparrow A. D. Multi-stemmed trees in montane rain forests: their frequency and demography in relation to elevation, soil nutrients and disturbance // Journal of Ecology. – 2009. – Vol. 97. – P. 472–483.
Benradje A., Bouazza M. and Boucherit H. Diversité floristique du peuplement à Pistacia atlantica Desf. dans la région de Béchar (Sud- ouest algérien) // Mediterranea. – 2012. – Vol. 23. – P. 66–89.
Biodiversity Support Program. Priority-setting in conservation: a new approach for Crimea: results of the conservation needs assessment in Crimea, supported by the Biodiversity Support Program. – Washington, D.C.: BSP, 1999. – 257 p.
Bychenko T. M. Methods of population monitoring of rare and endangered plant species of the Baikal region. – Irkutsk: Irkutsk State Pedagogical University Press, 2008. – 164 p. [In Russian]
Carpenter A., Elzinga C., Salzer D., Willoughby J. Measuring and monitoring plant populations // Journal of Range Management. – 1999. – Vol. 52. – 544 p.
Chernyshova E. B., Bondareva L. V., Alexandrov V. V., Alexeeva K. A. Status of Pistacia mutica Fisch. & C.A. Mey. populations in the region of Sevastopol // Terrestrial and marine ecosystems of the Black Sea region and their protection: Collection of abstracts of the scientific-practical school-conference (Novorosijsk, April 23–27, 2018). – Sevastopol: Federal State Budgetary Scientific Institution Institute of Natural and Technical Systems, 2018. – P.161–163. [In Russian]
Chistyakova A. A. Life forms and their spectra as indicators of the species state in the community (on the example of broad-leaved trees) // Bulletin of Moscow Society of Naturalists. Biological series. – 1988. – Vol. 93, N 6. – P. 93–105. [In Russian]
Coenopopulations of plants (basic concepts and structure) / [Eds. A. A. Uranov, T. I. Serebryakova]. – Moscow: Nauka, 1976. – 217 p. [In Russian]
Coenopopulations of plants (development and relationships) / [Eds. T. I. Serebryakova]. – Moscow: Nauka, 1977. – 131 p. [In Russian]
Czerepanov S. K. Vascular plants of Russia and adjacent states (the former USSR). – Cambridge: Cambridge University Press, 1995. – 516 p.
Desfontaines R. L. Flora atlantica; sive historia platarum quae in Atlante, agro tunetano et algeriensi crescunt.
Vol. 2. – Paris: L. G. Desgranges, 1799–1800. – 364 p.
El Zerey-Belaskri A. Taxonomic and botanical retrospective review of Pistacia atlantica Desf. (Anacardiaceae) // Arabian Journal of Medicinal & Aromatic Plants. – 2019. – Vol. 5, N 3. – P. 47–77.
Faouzi K., Rharrabti Y., Dardour M. Délimitation des peuplements du pistachier de l’Atlas (Pistacia atlantica Desf.) dans la région orientale du Maroc par le GPS combine SIG // Algerian journal of arid environment. – 2015. – Vol. 5, N 1. – P. 32–39.
Fischer F. G., Meyer A. Enumeratio plantarum quas in Provincia Talysch collegit // Bulletin de la société impériale des naturalistes de Moscow. – 1838. – Vol. 4. – P. 338–339.
Fortin M., Van Couwenberghe R., Perez V., Piedallu C. Evidence of climate effects on the height-diameter relationships of tree species // Annals of Forest Science. – 2019. – Vol. 76, N 1.
Ginsberg P. Afforestation in Israel: a source of social goods and services // Journal of Forestry. – 2000. – Vol. 98, N 3. – P. 32–36.
Glazkova E. A., Liksakova N. S. New and rare vascular plant species of the Kuril islands: distribution, ecology, and population status // Contemporary Problems of Ecology. – 2021. – Vol. 14, N 2. – P. 128–137.
Gourine N., Yousfi M., Bombarda I., Nadjemi B., Stocker P., Gaydou E. M. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria // Industrial Crops and Products. – 2010. – Vol. 31, N 2. – P. 203–208.
Green Data Book of the Ukraine / [ed. Didukh Ja. P.]. – Kyiv: Alterpress, 2009. – 448 p. [in Ukrainian]
Hosseini A., Pourhashemi M., Aazami A. Investigation on seedling emergence from direct seedings of Quercus persica, Pistacia atlantica and Acer cineracens in natural conditions of Ilam, Dalab Forests // Ecology of Iranian Forest. – 2021. – Vol. 9, N 17. – P. 41–48.
Isayeva Sh. Q. Population status of the rare species of the mud volcanoes in the Greater Caucasus // Proceedings of the Mordovia State Nature Reserve. – 2022. – Vol. 30. – P. 69–77.
Kafkas S. Phylogenetic analysis of the genus Pistacia by AFLP markers // Plant Systematics and Evolution. – 2006. – Vol. 262, N 1/2. – P. 113–124.
Kesslera M., Böhner J., Kluge J. Modelling tree height to assess climatic conditions at tree lines in the Bolivian Andes // Ecological modelling. – 2007. – Vol. 207, N 2–4. – P. 223–233.
Koba V. P., Zhigalova T. P. Germination of seeds of Pinus pallasiana (Pinaceae) and early succession grassy plant species in different types of substratium from wood detritus // Rastitelnye resursy. – 2014. – Vol. 50, N 1. – P. 33–39. [In Russian]
Kricsfalusy V. V., Mező-Kricsfalusy G. M. Population biology of plants: manual for higher school students of biology. – Uzhgorod: Univ. Press, 1994. – 80 p. [in Ukrainian]
Labdelli A., Zemour K., Simon V., Cerny M., Adda A., Merah O. Pistacia atlantica Desf., a source of healthy vegetable oil // Applied Sciences. – 2019. – Vol. 9, N 12. – 2552.
Letukhova V. Ju., Potapenko I. L., Kusnetsov M. E. The terebinth population (Pistacia mutica Fisсh. & C. A. Mey.) in the Besh-Tash valley (South-East Crimea) // Nature Conservation Research. – 2016. – Vol. 1, N 2. – P. 11–18. [In Russian]
Lines E. R., Zavala M. A., Purves D. W., Coomes D. A. Predictable changes in aboveground allometry of trees along gradients of temperature, aridity and competition // Global Ecology and Biogeography. – 2012. – Vol. 21. – P. 1017–1028.
Litvinskaya S. A. Terebinth – Pistacia mutica Fisch. et C.A. Mey. // Red Data Book of Russian Federation (plants and fungi). – Moscow: KMK Scientific Press Ltd, 2008. – P. 58–59. [In Russian]
Mahjoub F., Akhavan R. K., Yousefi M., Mohebbi M., Salari R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology // Journal of medicine and life. – 2018. – Vol. 11, N 3. – P. 180–186.
Matveenko N. V., Rubtzova T. A. Using the method of coenopopulations observation for monitoring rare species of vascular plants // Rarities of the Volga basin flora: Participants reports of the Russian scientific conference (Tolyatti, October 12–15, 2009). – Tolyatti: Kassandra, 2009. – P. 139–147. [In Russian]
Mazepa V. S., Devi N. M. Development of multistemmed life forms of Siberian larch as an indicator of climate change in the timberline ecotone of the Polar Urals // Russian Journal of Ecology. – 2007. – Vol. 38, N 6. – P. 440–443.
McCall A. C. Plant Population Ecology // Ecology / [ed. Gibson D.]. – New York: Oxford University Press, 2017.
Messaoud Y., Chen H. Y. H. The influence of recent climate change on tree height growth differs with species and spatial environment // PloS ONE. – 2011. – Vol. 6, N 2. – e14691.
Mirkin B. M., Naumova L. G., Solomeshch A. I. Modern vegetation science: a textbook. – Moscow: Logos Publ, 2001. – 264 p. [In Russian]
Nedoseko O. I. Life forms of boreal tree species of Salix L. // Vestnik of Lobachevsky University of Nizhni Novgorod. – 2012. – N 2–1. – P. 111–118. [In Russian]
Osmanova G. O., Zhivotovsky L. A. The ontogenetic spectrum as an indicator of the status of plant populations // Biology Bulletin. – 2020. – Vol. 47, N 2. – P. 141–148.
Potapenko I. L. Arboreal plants of aboriginal flora in planting of greenery in the east region of the South Crimean Coast // Optimization and Protection of Ecosystems. – 2010. – Vol. 2, N 21. – P. 30–41. [In Russian]
Potapenko I. L., Klimenko N. I., Letukhova V. Yu., Klimenko O. E. Trees and shrubs of native flora in green areas of the South-East coast of the Crimea // IOP Conference Series: Earth and Environmental Science. – 2021. – Vol. 723. – 022063.
POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. – 2022. – Available from: http://www.plantsoftheworldonline.org (accessed 01.10.2022)
Pulliam H. R. Sources, sinks and population regulation // American Naturalist. – 1988. – Vol. 135. – P. 652–661.
R Core Team. R: A language and environment for statistical computing. Version: 18 May 2021. – 2021. – Available from: http://www.R-project.org (accessed 01.05.2021)
Rabotnow T. A. Phytocoenology. – Moscow: Moscow university press, 1992. – 352 p. [In Russian]
Rebriev Ju. A., Sokolova T. A. Populations’ status of terebinth (Pistacia mutica) in some protected areas of Sevastopol // Terrestrial and marine ecosystems of the Black Sea region and their protection: Collection of abstracts of the II All-Russian scientific-practical school-conference (Kurornoye, September 28 – October 02, 2020). – Sevastopol: Federal State Budgetary Scientific Institution Institute of Natural and Technical Systems. – 2020. – P. 189–190. [In Russian]
Rezaeyan S., Pourmajidian M., Jalilvand H., Parsakhoo A. Growth parameters of Pistacia atlantica Desf under different soil conditions in Iran // African Journal of Plant Science. – 2009. – Vol. 3, N 9. – P. 184–189.
Shatko V. G., Mironova L. P. Synopsis of Tepe-Oba Ridge Flora (the Crimea) // Bulletin of the Main Botanical Garden. –2011. – Vol. 197. – P. 43–71. [In Russian]
Shevchenko S. V., Vasilieva Е. А. Features of the reproduction of Pistacia mutica in the Crimea // Proceedings of Nikita botanical garden. – 1992. – Vol. 113. – P. 45–51. [In Russian]
Shilovskaya E. A. Reproduction of Pistacia atlantica Desf in the Mountain Crimea // Scientific works of the SNBG. – 2018. – Vol. 147. – P. 71–73. [In Russian]
Shilovskaya E. A., Goncharenko V. V. The current state of genetic reserves of Pistacia mutica in south-west part of Mountain Crimea // Ekosistemy. – 2016. – Vol. 8, N 38. – P. 73–77. [In Russian]
Tanentzap A. J., Mountford E. P., Cooke A. S., Coomes D. A. The more stems the merrier: advantages of multi- stemmed architecture for the demography of understorey trees in a temperate broadleaf woodland // Journal of Ecology. – 2012. – Vol. 100. – P. 171–183.
The IUCN Red List of Threatened Species. Version 2022-1. – 2022. – Available from: https://www.iucnredlist.org (accessed 14.06.2022)
Uranov A. A. Age spectrum of phytopopulation as a function of the time of energy wave processes // Biological Sciences. – 1975. – Vol. 2. – P. 7–34. [In Russian]
Way D. A., Oren R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data // Tree Physiology. – 2010. – Vol. 30, N 6. – P. 669–688.
Yantzev A. V. Algorithms for applying statistical criteria. Statistical tables and formulas: manual for biology students. – Simferopol, 2007. – 76 p. [In Russian]
Yarysh V. L., Rogovoy V. I., Shvez Yu. P., Shilovskaya E. A. Taxation structure of Pistacia mutica plantings in Crimea // Proceedings of the Saint Petersburg forestry research institute. – 2019. – N 1. – P. 15–24. [In Russian]
Yarysh V. L., Yarysh G. E. Analysis of the undergrowth plantings of terebinth (Pistacia mutica) in the Karadag nature reserve // Scientific Notes of V. I. Vernadsky Crimean Federal University. Biology. Chemistry. – 2020. – Vol. 6, N 72. – P. 291–303. [In Russian]
Yasinskaya O. I., Kostina M. V., Barabanshchikova N. S. Structural and ecological peculiarities of Acer negundo L. life forms // Systematic and floristic studies of Northern Eurasia: Proceedings of the II International Conference (to the 90th anniversary of the birth of Professor A. G. Yelenevsky) (Moscow, December 05-08, 2018). – Moscow: MPSU, 2018. – Vol. 3. –P. 146–150. [In Russian]
Zhivotovsky L. A. Ontogenetic states, effective density, and classification of plant populations // Russian Journal of Ecology. – 2001. – Vol. 32, N 1. – P. 1–5.
Zhou Y., Lei Z., Zhou F., Han Y., Yu D., Zhang Y. Impact of climate factors on height growth of Pinus sylvestris
var. mongolica // PLoS ONE. – 2019. – Vol. 14, N 3. – e0213509.
Zohary M. A monographical study of the genus Pistacia // Palestine Journal of Botany. Jerusalem Series. – 1952. – Vol. 5. – P. 187–228.
Летухова В. Ю., Потапенко И. Л. Pistacia atlantica (Anacardiaceae) в Юго-Восточном Крыму: характеристика популяций, современное состояние, охрана // Экосистемы. 2022. Вып. 31. С. 5–19.
В статье приведены результаты мониторинга популяций реликтового средиземноморского охраняемого вида Pistacia atlantica Desf, включенного в Красную книгу Российской Федерации. В Крыму P. atlantica встречается в горах Южного Берега Крыма (от Балаклавы до Карадага) в прибрежном и нижнем горно-лесном поясах до 400 м н.у.м, где образует редкие реликтовые растительные сообщества. Основным фактором угрозы для этих сообществ является антропогенное воздействие на прибрежные ландшафты. В настоящее время древние средиземноморские леса сократились до небольших рощ, разбросанных по побережью, и поэтому они нуждаются в постоянном мониторинге и охране. Полевые исследования проводились в 2018–2020 годы. Всего было выделено и обследовано семь популяций, из которых четыре естественного происхождения и три искусственного происхождения. На каждом участке закладывали пробные площади, на которых подсчитывали общее количество растений P. atlantica и определяли их возрастные состояния. Также были измерены биоморфологические показатели генеративных растений: высота дерева, количество стволов, диаметры ствола у корневой шейки и на высоте 1,3 м. На каждой площадке изучали онтогенетическую структуру популяции, рассчитывали индекс возрастности (Δ), индекс средней эффективности (ω) и индекс восстановления, определяли тип популяций (по классификации (∆–ω)). Популяции естественного происхождения были определены как молодые (индекс возрастности 0,17–0,24; индекс средней эффективности 0,45–0,56; индекс восстановления 43,2–158,7 %). Они имели левосторонний спектр с преобладанием прегенеративных особей. Искусственные популяций оказались зреющими или зрелыми (индекс возрастности 0,30–0,51; индекс средней эффективности 0,70–0,76; индекс восстановления 12,8–20,5 %). Они имели либо центрированный (преобладали молодые или средневозрастные генеративные особи), либо правосторонний (преобладали старые генеративные особи) спектры. Средняя высота деревьев в популяциях варьировала от 3,0 до 5,5 м. Мы обнаружили статистически значимую положительную корреляцию между этим параметром и годовым количеством осадков. Среднее количество стволов у деревьев в популяциях варьировало от 1,4 до 2,4. Этот параметр отрицательно связан с годовым количеством осадков. С другой стороны, влияние температуры на морфологические параметры деревьев было статистически незначимым. Состояние всех исследованных популяций было признано удовлетворительным, общее фитосанитарное состояние деревьев также не вызывает опасений. Было обнаружено новое место произрастания
P. atlantica и, таким образом, ареал этого вида расширился до района города Феодосии. Главным фактором риска для этого вида в Юго-Восточном Крыму является сокращение площадей произрастания за счет увеличения рекреационной нагрузки на природные территории.
Ключевые слова: влияние климата, Крым, мониторинг, онтогенетическая структура, редкие виды, фисташка туполистная.
Поступила в редакцию 05.10.22 Принята к печати 10.11.22

