Ekosistemy, 30: 5–21 (2022) https://ekosystems.cfuv.ru
УДК [574.587:595.142.2](262.5.04)
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
Boltachova N. A., Lisitskaya E. V., Revkov N. K., Podzorova D. V.
A. O. Kovalevsky Institute of Biology of the Southern Seas of RAS
Sevastopol, Russia
A total of 75 polychaete species (Polychaeta) were recorded as a result of the macrozoobenthos surveys carried out in 2007–2018 in Karkinit Bay, northwestern Black Sea. The previously known taxonomic list of polychaetes was extended by adding 46 new species. To date, 86 polychaete species belonging to 32 families have been identified in Karkinit Bay throughout the period of zoobenthos studies (1930–2018). In terms of number of species, the most widely represented families were the Phyllodocidae (10 species), Syllidae (11), Spionidae (8), and Nereididae (7). During our study, the average population density of polychaetes ranged within 498–1420 ind.·m−2, with a maximum of 17708 ind.·m−2. Differences in the structure and abundance of polychaetes were found between the shallow-water eastern (Zabakalsky) part and the deeper western part of Karkinit Bay. The polychaete taxocene of the shallow waters in the Zabakalsky part was significantly affected by the increase in water salinity due to the damming of the North Crimean Canal in 2014. In 2007– 2013, Hediste diversicolor dominated in abundance at stations with a salinity lower than 10 ‰, reaching 2313 ind.·m−2. The population density of H. diversicolor showed an inverse relationship with water salinity. A change of dominant species occurred in the Zabakalsky part in 2016–2018, and the average density of polychaetes decreased 2.7-fold. No significant changes were observed in the western, deep-water part of Karkinit Bay after the closure of the canal. The maximum density values (up to 16740 ind. m−2) were recorded for Melinna palmata in 2007–2013 and for Prionospio cf. cirrifera (up to 2984 ind.·m−2) in 2016–2018. The species Heteromastus filiformis was categorized as leading all over the Bay area.
Key words: Annelida, polychaetes, Hediste diversicolor, zoobenthos, Black Sea.
INTRODUCTION
In the mid 20th century, V.A. Vodyanitsky (1949) identified five natural regions based on an analysis of physico-geographical, hydrological, and biological characteristics of the Black Sea coastal zone of Crimea: Karkinit, Yevpatoria–Sevastopol, South Coast, Feodosia, and Kerch. In recent decades, the benthic fauna of each of these regions has been exposed, to a greater or lesser extent, to the negative anthropogenic pressure on both the global and local scales. The consequences of these impacts have not been fully understood. Karkinit Bay is the largest body of water in the Black Sea and one of its most productive areas. This explains the interest in the study of its inhabitants exposed to changing environmental conditions. Nevertheless, the fauna of this Bay and, especially, its shallow-water parts has not been studied in sufficient detail.
A survey of Karkinit Bay conducted in the early 20th century and then studies in the 1930s showed that the macrozoobenthos of the Bay is extremely diverse and rich in quantitative terms (Zernov, 1913; Arnoldy, 1949). The results obtained in the 1950s confirmed the available data concerning the distribution of the benthic fauna in the study region (Vinogradov, 1959; Zakutsky, 1962; Zakutsky & Vinogradov, 1967). Substantial changes in the benthos of Karkinit Bay probably began in the late 1970s, when suffocation events, first recorded from the northwestern Black Sea (NWBS), also began to occur here (Povchun, 1990). The continuing transformation of the benthos indicated the siltation and pollution of the Bay (Povchun, 1992).
Since the 1970s, one of the factors that exerted serious effects on the biota of Karkinit Bay was the construction (1961–1971) of the North Crimean Canal, which was accompanied by the development of irrigation agriculture and a system of fish rearing ponds. This inevitably affected the structure and distribution of the local benthic fauna. It should be noted that the benthos surveys of the Bay in the 1980s were carried out mainly in the central and western, relatively deep-water parts (Povchun, 1990, 1992; Zolotarev et al., 1991; Terentyev, 2002). However, information about the zoobenthos in the eastern, shallow-water part of Karkinit Bay for that period was extremely scarce
ISSN 2414-4738 Published by V. I. Vernadsky Crimean Federal University, Simferopol
Boltachova N. A., Lisitskaya E. V., Revkov N. K., Podzorova D. V.
![]()
and was limited to data collected almost 70 years before, when there had been no freshwater runoff into the Bay (Arnoldy, 1949). Currently (since 2014), the operation of the North Crimean Canal is discontinued and, accordingly, the freshwater discharge into Karkinit Bay has actually reduced to a level that existed 50 years ago. This suggests dramatic changes in the benthos of the parts of the Bay previously exposed to severe freshening. However, there is still a significant lack of such information.
It should also be noted that all studies of the 1930s and 1980s considered zoobenthos in general, without any dedicated investigations into the polychaete (Polychaeta) fauna.
Our work aimed to study the fauna of polychaete worms in Karkinit Bay in the early 21st century, and also analyze the available data on the taxonomic structure of this group for all the major periods of zoobenthos research in this region. Special attention is paid to the composition and structure of the polychaete taxocene during the period of maximum exposure to the discharge from the North Crimean Canal and freshening of the water in the Bay.
MATERIAL AND METHODS
In our analysis, we used the materials of the benthic surveys conducted by the Benthos Ecology Department, A.O. Kovalevsky Institute of Biology of the Southern Seas RAS, in the western and Zabakalsky areas of Karkinit Bay, NWBS, in 2007–2013 and 2016–2018 (Fig. 1, Table 1).
Benthic material from the eastern (apex) part of the Bay was collected in the summer seasons of 2007, 2008, and 2009. Sampling was carried out by SCUBA divers using a manual grab sampler (S=0.04 m2) in duplicates. A total of 67 stations were sampled within a depth range of 0–9 m. In 2018, macrozoobenthos was collected at 25 of these stations at depths of 0–5 m with the use of the same sampling gear. Collected sediments were washed through sieves with a 0.5 mm mesh.
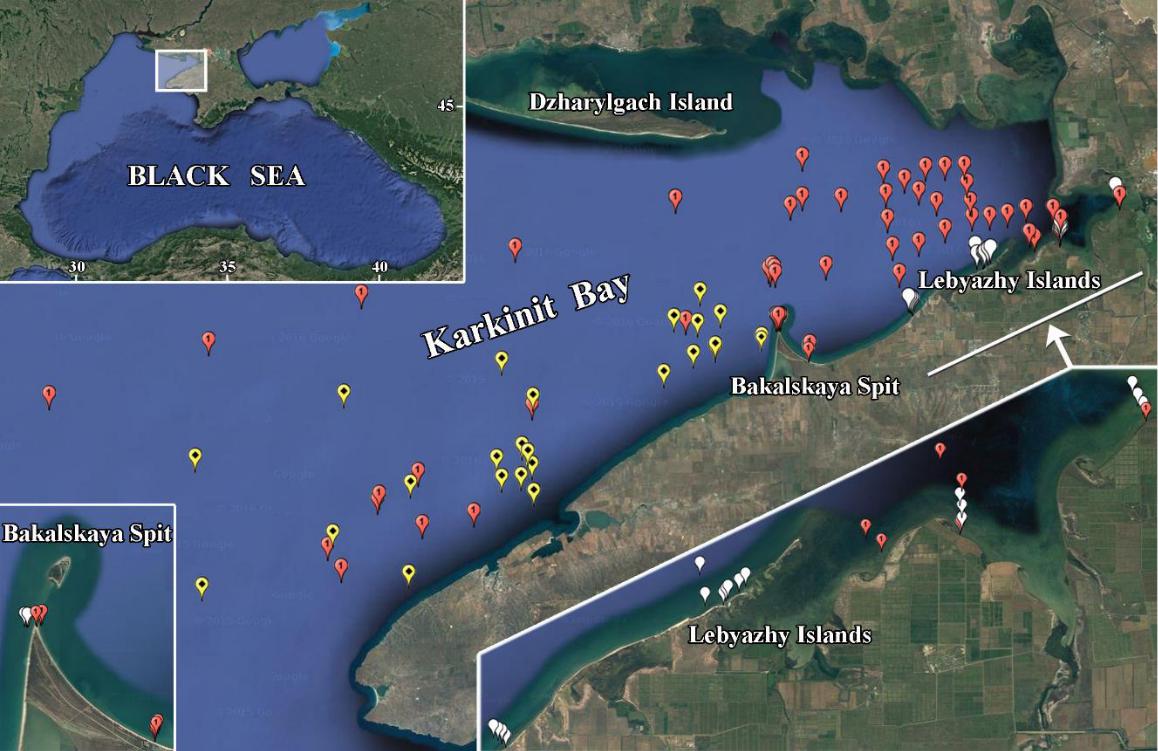
Fig. 1. Benthic studies in Karkinit Bay in 2007–2018
Red dots indicate sampling stations in 2007–2013; yellow dots, in 2016–2018; white dots, in 2008 and 2018.
Рис. 1. Исследования бентоса в Каркинитском заливе в 2007–2018 годах
Красными точками обозначены станции отбора проб в 2007–2013 годах; желтыми точками – в 2016– 2018 годах; белыми точками – в 2008 и 2018 годах.
6
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
![]()
Table 1
Distribution of the sampling stations in Karkinit Bay depending on study periods, areas, and
salinity ranges of the near-bottom water layer
Таблица 1
Распределение станций отбора проб в Каркинитском заливе в зависимости от периодов
исследований, районов и диапазонов солености придонного слоя воды
| 2007–2013 | 2016–2018 | |||||
| Area | Location of areas, depth | Number of | Salinity, ‰ | Number of | Salinity, ‰ | |
| stations | stations | |||||
| Apex to Lebyazhy Islands, | 14 | 1.5–10.3 | 15 | 20.4–27.3 | ||
| 0–3 m | ||||||
| Zabakalsky | Lebyazhy Islands to | |||||
| Bakalskaya Spit, | 30 | 16.2–18.9 | 10 | 18.4–19.8 | ||
| 0–3 m | ||||||
| middle of the Bay, | 23 | 18.1–18.7 | – | – | ||
| 3–9 m | ||||||
| Western | 10–41 m | 19 | 17.76–18.03 | 23 | 18.14–18.38 | |
![]()
![]()
Additionally, qualitative samples of periphyton on frames (S=0.04 m2, sieve with a 0.5 mm mesh size) were collected in triplicates at 10 stations within a depth range of 0–2 m in the summer seasons of 2005 and 2007. The central and western parts of the Bay were surveyed during the cruises #70 and #72 aboard the R/V Professor Vodyanitsky in 2011 and 2013. The material was collected using an “Okean-50” grab sampler (S=0.25 m2) at 19 stations within a depth range of 10–41 m. In the southwestern part of the Bay, the material was collected at 23 stations during the cruises #84, #86 (in 2016), and #96 (in 2017) aboard the R/V Professor Vodyanitsky. At each station, 1–2 bottom sediment samples were taken. Sediments were washed through a system of sieves with a minimum mesh size of 1 mm. The material was fixed in a 4 % neutral formalin solution. Water temperature and salinity in the near-bottom layer were measured at all stations (Table 1).
The following publications were used for the taxonomic identification of the material collected:
Vinogradov & Losovskaya (1968), Kiseleva (2004).
Frequency of species occurrence was calculated by the following formula:
![]()
where a is the number of stations where the species was encountered; n is the total number of stations in the study area.
Species with an occurrence frequency of 50 % or more were categorized as leading; species with an occurrence frequency of 25–50 %, as characteristic; and species found at less than 25 % of the sampled stations, as rare (Vorobyov, 1949). To assess the similarity of polychaete species compositions between different study years, we used the Czekanowski–Sørensen index:
![]()
where c is the number of species common for both lists; a and b are the numbers of species in each of the lists.
Multivariate statistical algorithm was used to assess the structural organisation of polychaetes taxocene. Determining the characteristic species of the selected spatial polychaetes complexes was realized based on their contribution to the intra- and intercomplex similarity (SIMPER analysis, PRIMER-6 software package) according to the non-transformed values of their abundance (Clarke, Gorley, 2001). The Bray-Curtis statistics (Bray, Curtis, 1957) was used as a measure for similarity. In the comparative prognostic estimation of expected species number we used commonly applied Chao-
7
Boltachova N. A., Lisitskaya E. V., Revkov N. K., Podzorova D. V.
![]()
2, Jacknife-1 and Jacknife-2 estimators (Foggo et al., 2003), calculated in PRIMER’s Species-Accum plot routine. Possible differences between polychaetes complexes were tested for significance using analysis of similarity (ANOVA) in STATISTICA-6 software package.
Description of the study region. Karkinit Bay occupies a significant area in the eastern NWBS between the northwestern Crimea coast and the mainland. It is the largest, but relatively shallow Bay of the Black Sea. Its extent in the estuarine part from north to south (from the Tendrovskaya Spit to Cape Tarkhankut) is about 130 km; from west to east, 140 km. On the basis of its geomorphological structure, the Bay can be conditionally divided into the main western part (with depths of up to 45
- and the shallow-water eastern part (with depths of up to 9–10 m). The border between them can be drawn along the line from the Bakalskaya Spit to the Bakalskaya Bank (with depths of up to 3 m) and further to Dzharylgach Island (Kondratiev, 2018). The bottom in the coastal zone is composed mainly of sands with clay outcrops; the southern shore of the Bay is mostly rocky. At depths greater than 20–25 m, sandy sediments are replaced by silty shell debris and aleurite/pelite silts (Arnoldy, 1949; Povchun, 1990) (Fig. 2).

Fig. 2. Karkinit Bay, the upper part of the Zabakalsky area
a – coastal zone; b – a site of the bottom with macrophyte beds.
Рис. 2. Каркинитский залив, вершинная часть Забакальского района
a – прибрежная зона; b – участок дна с зарослями макрофитов.
The coast of the Bay lacks rivers and is characterized by a low amount of precipitation. The hydrological regime shows a pronounced pattern of seasonal and year-to-year variations, especially in the eastern part. In winter, a significant part of the Bay freezes up; in summer, the water warms up to 26.9 °C, and even up to 29–30 °C in the shallow-water area. The variation in the parameters is largely related with the uneven inflow of the shelf water into the Bay. Along the northern coast, the Bay receives the freshened (with a salinity lower than 17 ‰) water from the northwestern shelf; a more saline (over 18 ‰) water of the open part of the sea comes from the south and southwest (Pukhtyar, 2007). The inflow of the freshened water into the Bay begins in early spring, intensifies in summer, and weakens in early autumn. In winter, the Rim Current increases, which causes the almost complete “isolation” of Karkinit Bay, and the inflow of this water stops. The water salinity over the major part of the Bay ranges within 13.87–18.74 ‰, rising in some years to 19 ‰, and even to 20.8 ‰ in the shallow part (Pukhtyar et al. 2003). Due to the intensive water warming and evaporation, the salinity in the shallow part becomes usually significantly higher than in the rest of the Bay by late summer. The entry of water into the eastern (Zabakalsky) part of the Bay is largely reduced due to its isolation. The water from the main part of the Bay can enter the eastern part through the Bakalsky Strait only with westerly and southwesterly winds. As a result, the eastern, shallow part of the Bay in the warm season can have an almost complete lack of water exchange with the main part of the Bay for up to two months. In the western, deep-water part, however, the time interval of renewal of the upper water layer is no longer than two weeks in the spring–summer period (Pukhtyar et al., 2003; Pukhtyar, 2007).
8
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
![]()
After the North Crimean Canal was put into service, seasonal discharges of fresh water from irrigation canals, in particular from those in its Razdolnenskaya branch (in 1984), caused dramatic changes in the hydrological and hydrochemical parameters of the waters in Karkinit Bay, which became especially pronounced in the eastern part, behind the Bakalskaya Spit (Yurovsky, 2001). In the upper (apex) part of the Bay, areas of significant freshening with salinities of 0.99–2.72 ‰ appeared at the sites of discharge of the Dnieper water from rice paddy fields and fish rearing ponds (Kondratiev, 2018). During the period of our study in 2007–2009, the zone of freshening of up to 1.5–10.3 ‰ was observed to extend from the apex of the Bay to the Lebyazhy Islands (Revkov et al., 2010).
The eastern, shallow part of the Bay, being subject to a high anthropogenic pressure, is of particular interest as regards its hydrochemistry and ecology. Technogenic radionuclides 137Cs and 90Sr continuously entered the upper part of Karkinit Bay with the water discharged from the irrigation systems of the Crimean Peninsula (Polikarpov et al., 2008; Gulin et al., 2016). During our study in 2008, the polychlorinated biphenyl (PCB) content of bottom sediments in the shallow waters of the Zabakalsky part did not exceed the maximum permissible concentration (MPC), while the concentration of DDT compounds in the upper part of the Bay exceeded the MPC 1.6-fold (Malakhova et al., 2019).
Also, a significant factor in the water pollution is the discharges of wastewater from the chemical plants of Armyansk and Krasnoperekopsk, producing aniline dyes, sodium bicarbonate, titanium dioxide, sulfuric acid, and other substances, into the upper part of the Bay (Kondratiev, 2018). As a result, due to the presence of toxic substances in the water of the Bay, its ecological status in 2011 was evaluated as “catastrophic” (Sovga et al., 2011).
Thus, the remoteness of Karkinit Bay from the deep-water part of the sea, its shallow depths, the peculiar hydrological regime, and also the extreme anthropogenic pressure create specific conditions for the benthic fauna here that differ from those existing in other regions of the sea.
RESULTS
As a result of our study, we found a total of 75 species of polychaete worms belonging to 28 families in Karkinit Bay (Table 2). In terms of number of species, the most widely represented families were the Syllidae (11 species), Spionidae (8), Phyllodocidae (8), and Nereididae (7). Two species were recorded as non-native in the Black Sea: Sigambra tentaculata (Treadwell, 1941) and Polydora cornuta Bosc, 1802 (Boltachova et al, 2016).
Table 2
Taxonomic structure and frequency of occurrence (F, %) of polychaetes (Polychaeta)
in Karkinit Bay in the 20th and early 21st centuries
Таблица 2
Таксономический состав и частота встречаемости (F, %) полихет Каркинитского залива
в 20 и в начале 21 века
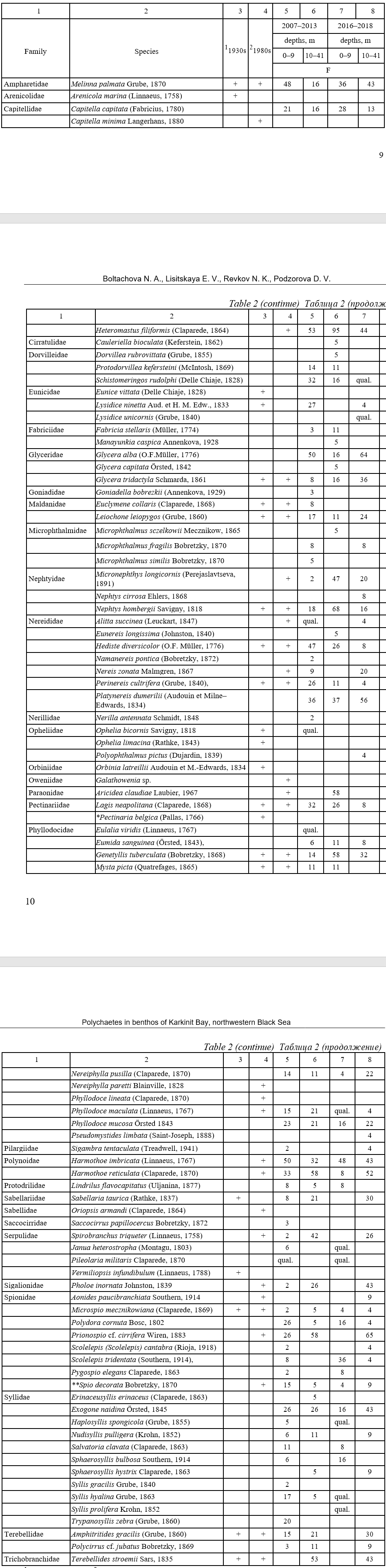
Note: 1 (Arnoldy, 1949); 2 (Zolotarev, Povchun, 1986; Povchun, 1990, 1992; Zolotarev et al., 1991); * the species was recorded in the 1950s (Vinogradov & Losovskaya, 1968); ** the species was earlier referred to as Spio filicornis (Müller, 1776) (Boltachova, Lisitskaya, 2019); “qual.” means that the species was found in qualitative samples.
In accordance with the geomorphological features of the Karkinit Bay, the polychaete taxocene was analysed in its two main areas: the Zabakalsky and western areas. At the same time, due to changes in hydrological conditions, in mentioned above sectors of the Bay the analysis was performed for time intervals: before and after 2014. Based on the subdivision of stations into 4 groups and the importance of polychaete species in the selected station complexes by their contributing most to the average intracomplex similarity (Table 3), the polychaete taxocene in the benthos of Karkinit Bay can be described by four complexes: I—Heteromastus filiformis + Hediste diversicolor, II— Nephtys hombergii + Heteromastus filiformis, III—Heteromastus filiformis + Prionospio cf. cirrifera
- Aricidea claudiae and IV—Platynereis dumerilii. Significant differences in the polychaete abundance were noted between complexes I and IV (p=0.0008), III and IV (p=0.017), in other cases differences were not significant (p>0.25).
The relatively low values of intracomplex similarity (14.21–20.27 %) indicate the high heterogeneity of polychaete assemblages and the possible problematic nature of our “artificial” subdivision of the whole taxocene into 4 complexes. However, a comparison of the already identified complexes with each other showed that in all cases the intercomplex similarity is lower than the intracomplex similarity (Fig. 3).
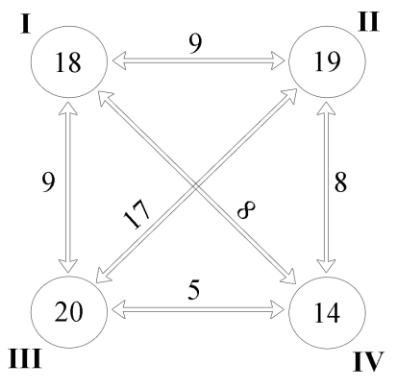
Fig. 3. Intra- and intercomplex similarity (Bray-Curtys Similarity) of the polychaete fauna of the Karkinit Bay
I–IV – polychaete complexes. The circles show values of similarity coefficients within the respective complexes, outside the circles – intercomplex similarity.
Рис. 3. Внутри- и межкомплексное сходство (Bray-Curtys Similarity) фауны полихет Каркинитского залива
I–IV – комплексы полихет. В кружках указаны значения коэффициентов сходства внутри соответствующих комплексов, вне кружков – межкомплексное сходство.
From these positions (quantitative distribution of various species), the subdivision of the polychaete fauna, taken by us as the basis, corresponding to the physical-geographical zoning of the water area of the Karkinit Bay, is not senseless.
Composition and structure of the polychaete fauna in Karkinit Bay in the period 2007– 2013. A total of 59 species representing 25 families were recorded from the shallow-water Zabakalsky area, and 46 species (22 families) were recorded from depths greater than 10 m in the western part of the Bay. The Czekanowski–Sørensen faunal similarity index for these areas amounted to 0.69.
In the Zabakalsky area (complex I – Heteromastus filiformis + Hediste diversicolor), the group of leading species (with an occurrence frequency of >50 %) was comprised of Heteromastus filiformis, Harmothoe imbricata, and Glycera alba. The group of characteristic species (with an occurrence frequency of 25–50 %) was comprised of 11 species: Hediste diversicolor, Harmothoe reticulata, Platynereis dumerilii, Shistomeringos rudolphi, Lagis neapolitana, Melinna palmata,
12
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
Table 3
The principal polychaetes species contributing most to the average similarity within the corresponding taxocoenotic complexes
Таблица 3
Виды полихет, вносящих наибольший вклад в среднее внутрикомплексное сходство выделенных таксоценотических комплексов
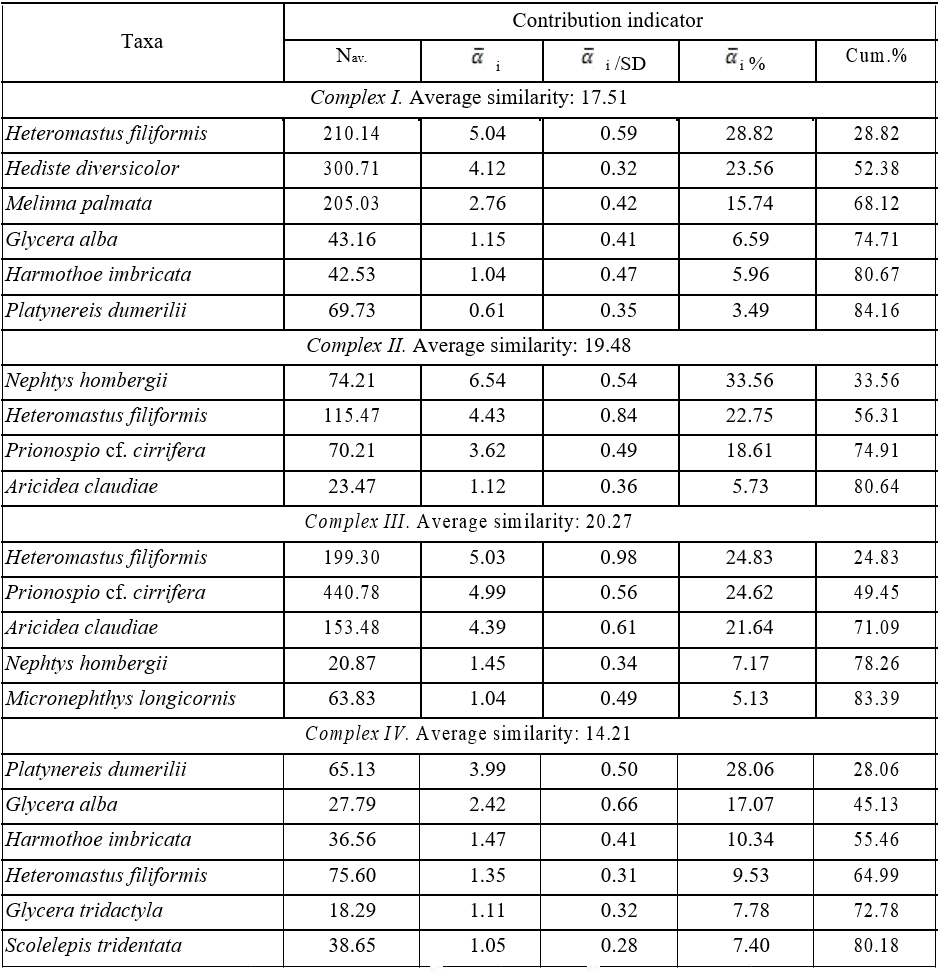
Note: Nav. – average abundance of polychaetes; ![]() i – absolute and
i – absolute and ![]() i % – the relative contributions of “i”– species to the average Bray-Curtis similarity within the complex; SD – standard deviation; Cum.% – total relative contribution of species to the average Bray-Curtis similarity within the corresponding groupings.
i % – the relative contributions of “i”– species to the average Bray-Curtis similarity within the complex; SD – standard deviation; Cum.% – total relative contribution of species to the average Bray-Curtis similarity within the corresponding groupings.
Lysidice ninneta, Perinereis cultrifera, Polydora cornuta, Exogone naidina, and Prionospio cf. cirrifera (Fig. 4). The highest population density values were recorded for Hediste diversicolor (with
- mean of 300 ind.·m−2 and a maximum of 2313 ind.·m−2), Melinna palmata (205 and 1038 ind.·m−2), and Heteromastus filiformis (210 and 950 ind.·m−2). Some species that dominated in macrophyte beds – Harmothoe reticulata, Trypanosyllis zebra, and Platynereis dumerilii – showed a relatively high density (56–67 ind.·m−2). The density of polychaetes in the Zabakalsky area ranged within 50–
13
Boltachova N. A., Lisitskaya E. V., Revkov N. K., Podzorova D. V.
5963 ind.·m−2, with an average of 1343±331 ind.·m− 2, and reached the maximum values at a depth of 5–8 m in the central part of the Zabakalsky area. Hediste diversicolor and Trypanosyllis zebra made the greatest contribution to the abundance (12–20 %)
During the same period (2011–2013), the leading species at depths of 10–41 in the western part of Karkinit Bay (complex II – Nephtys hombergii + Heteromastus filiformis) were as follows: Heteromastus filiformis, Nephtys hombergii, Prionospio cf.cirrifera, Aricidea claudiae, Genetyllis tuberculata, Harmothoe reticulata, and Terebellides stroemii. The group of characteristic species was comprised of eight ones: Micronephthys longicornis, Spirobranchus triqueter, Platynereis dumerilii, Harmothoe imbricata, Lagis neapolitana, Hediste diversicolor, Exogone naidina, and Pholoe inornata. The density of polychaetes in the western part of the Bay ranged widely within 56– 17708 ind.·m−2, with an average of 1420±1293 ind.·m−2. The maximum population density of polychaetes was recorded from a depth of 35 m, where Melinna palmata constituted 94 % of it. A very high density (more than 1000 ind.·m−2) was also observed at a depth of 11 m (at stations within the Small Phyllophora Field), where Heteromastus filiformis dominated. The highest average
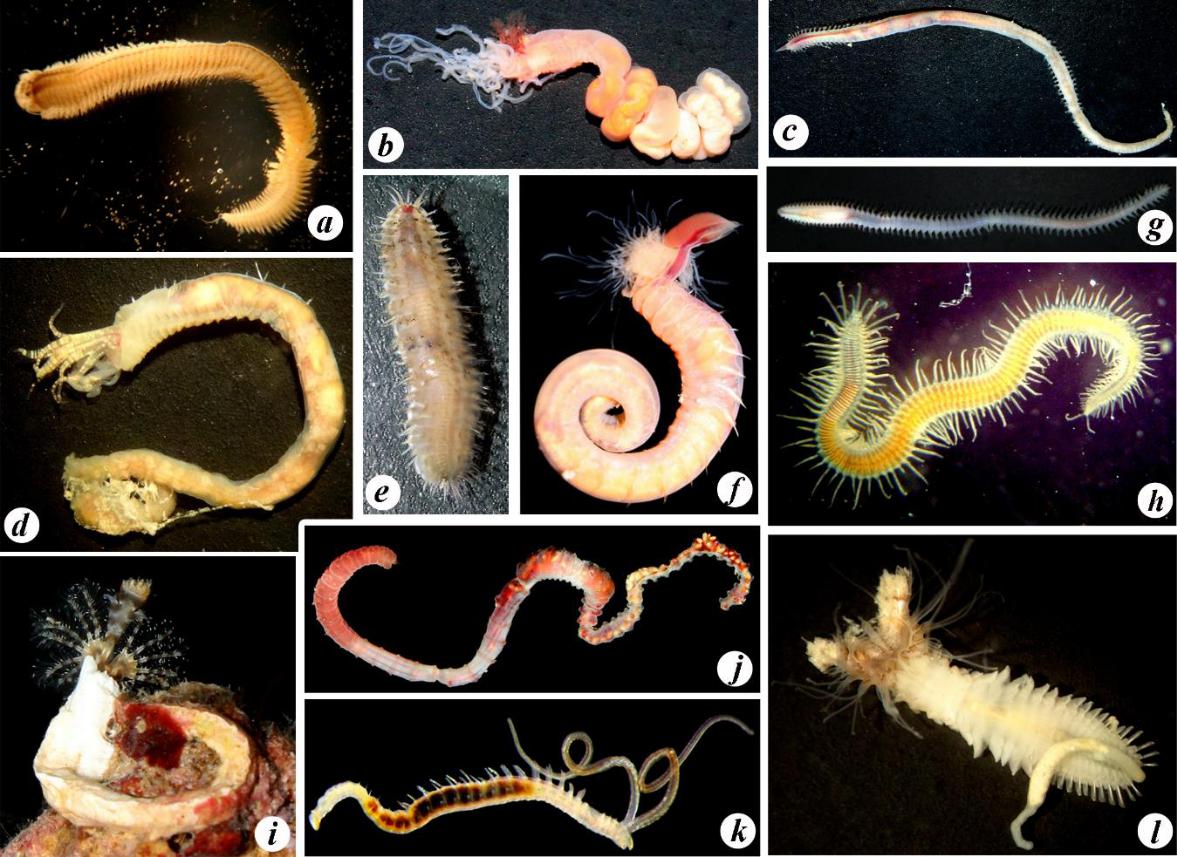
Fig. 4. Some polychaete species characteristic of Karkinit Bay
a – Hediste diversicolor; b – Amphitritides gracilis; c – Glycera alba; d – Melinna palmata; e – Harmothoe sp.; f – Terebellides stroemii; g –Nephtys hombergii; h – Trypanosyllis zebra; i – Spirobranchus triqueter; j – Heteromastus filiformis; k – Polydora cornuta; l – Sabellaria taurica (photos by A. A. Nadolny).
Рис. 4. Некоторые виды полихет, характерные для Каркинитского залива
a – Hediste diversicolor; b – Amphitritides gracilis; c – Glycera alba; d – Melinna palmata; e – Harmothoe sp.; f – Terebellides stroemii; g – Nephtys hombergii; h – Trypanosyllis zebra; i – Spirobranchus triqueter; j – Heteromastus filiformis; k – Polydora cornuta; l – Sabellaria taurica (фото А. А. Надольного).
14
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
![]()
densities for this area were recorded for Melinna palmata (914 ind.·m−2) and Heteromastus filiformis (115 ind.·m−2). The density values were also relatively high for Nephtys hombergii (74 ind.·m−2), Prionospio cf. cirrifera (70 ind.·m−2), and Harmothoe reticulata (48 ind.·m−2).
Composition and structure of the polychaete fauna in Karkinit Bay in the period 2016– 2018 after the closure of the North Crimean Canal. In the summer of 2018, a total of 41 polychaete species belonging to 17 families were recorded from the Zabakalsky area and 46 species belonging to 21 families from the western part of Karkinit Bay in 2016–2017. The Czekanowski– Sørensen faunal similarity index for these waters amounted to 0.61.
In 2016–2017, the group of leading species in the western Karkinit Bay at depths 10–41 m (complex III – Heteromastus filiformis + Prionospio cf. cirrifera + Aricidea claudiae) was comprised of Heteromastus filiformis, Nephtys hombergii, Micronephthys longicornis, Prionospio cf. cirrifera, Aricidea claudiae, and Harmothoe reticulata. The group of characteristic species was comprised of 14 ones (Table 2). The population density of polychaetes at the stations ranged within 124–5952 ind.·m−2, with an average of 1291±615 ind.·m−2. High density values were recorded for Prionospio cf. cirrifera (with a mean value of 440 ind.·m−2 and a maximum of 2984 ind.·m−2), Aricidea claudiae (153 and 1756 ind.·m−2), and Heteromastus filiformis (200 and 944 ind.·m−2). The maximum polychaete density values (4196 and 5952 ind.·m−2) were recorded from a bottom area with silted shell debris located within a depth range of 20–22 m slightly north of Cape Tarkhankut; the density of the spionid Prionospio cf. cirrifera accounted for 50–65 % of these values.
The group of leading species in the Zabakalsky area in 2018 (complex IV – Platynereis dumerilii) was comprised of Platynereis dumerilii and Glycera alba; the group of characteristic species was comprised of Harmothoe imbricata, Heteromastus filiformis, Melinna palmata, Scolelepis tridentata, Glycera tridactyla, Genetyllis tuberculata, and Capitella capitata. The density of polychaetes at the stations ranged within 25–2338 ind.·m−2, with an average of 498±219 ind.·m−2. Relatively high densities were recorded for Heteromastus filiformis (with a mean of 73 ind.·m−2 and a maximum of 1213 ind.·m− 2), Platynereis dumerilii (65 and 288 ind.·m–2), and Polydora cornuta (48 and 600 ind.·m−2). High polychaete density values (1363–1400 ind.·m−2) were recorded at the stations located in the very apex of the Bay at a salinity of 20.4–27.3 ‰; the density of Polydora cornuta accounted for 40–43 % of these values.
DISCUSSION
In the first, the most extensive study of the benthic fauna in Karkinit Bay in the 1930s that covered the entire area from the water’s edge to depths of 30 m, not all but the most common polychaete species were identified in the Annelida group (Arnoldy, 1949). This probably explains the fact that only 22 species from 16 families were recorded from the samples then. The studies in the 1980s covered depths of 5–40 m, but mainly the western area with depths greater than 10 m was surveyed. At that time, 31 species (17 families) were found (Zolotarev, Povchun, 1986; Povchun, 1990, 1992; Zolotarev et al., 1991). In 2007–2018, we surveyed depths from 0 to 40 m, with, however, twice as many stations performed in the shallow-water Zabakalsky part of the Bay as in the western area. A total of 75 polychaete species representing 28 families were recorded.
Thus, we have extended the taxonomic list of polychaetes from Karkinit Bay by adding 46 species new to the region. One of these species, Heteromastus filiformis, was first discovered in Sevastopol Bay in the 1920s and was not known for other areas of the Black Sea at that time (Yakubova, 1930). Subsequently, the species distributed widely and became common in sandy/silty habitats, where its maximum abundance reached 227 ind.·m− 2 (Marinov, 1977; Kiseleva, 2004). In our collections, this species was categorized as leading in both study periods and in both areas: in the shallow-water Zabakalsky part and in the deeper western part of Karkinit Bay. Its abundance reached 950 ind.·m−2 in 2007–2013 and 1213 ind.·m−2 in 2016–2018.
Two species, Polydora cornuta and Sigambra tentaculata, are relatively new to the Black Sea. They were introduced in the basin in the 1960s and were not widely distributed there in the 1980s (Surugiu, 2012; Boltachova et al., 2021). Eleven of the species found only in our studies belong to the family Syllidae. The Black Sea Syllidae are mainly small-sized species, mostly represented in
15
Boltachova N. A., Lisitskaya E. V., Revkov N. K., Podzorova D. V.
![]()
the meiobenthos, and, therefore, they could be overlooked in samples of previous researchers. Furthermore, they are more typical for shallow waters, which were studied more in detail in 2007– 2018.
A total of 86 polychaete species belonging to 32 families have been found in Karkinit Bay throughout the period of zoobenthos research since 1934 (Table 2). Six species were mentioned only in the first study period (1930s) and five only in the second one (1980s). These are mainly such species as Arenicola marina, Nereiphylla paretti, Phyllodoce lineata, Pectinaria belgica, Orbinia latreillii, and Galathowenia sp. which rarely occur in the Black Sea (Kiseleva, 2004). A total of 13 species were common in all three study periods (in 1930s, 1980s, and 2007–2018); of them, 10 are currently leading or characteristic in the benthos of the Bay.
A prognostic estimation of the expected species richness of the polychaete taxocene in the region of Karkinit Bay, calculated on the basis of our data (2007–2018), showed as follows (Fig. 5). At the 116 th sample/station (out of 135 sampled in the study region), the Chao2 curve forms a plateau, reaching a value of 98 (SD=16) polychaete species. Close levels of the theoretically expected species richness of the polychaete taxocene at the end points of the curves are achieved using the estimators Jacknife1 (93 species) and Jacknife2 (103 species). The estimate of the theoretically expected number of polychaete species in the waters of Karkinit Bay, obtained this way, is only by approximately 20 species higher than the level of 76 species that we actually recorded in 2007–2018. The total number of members of this group (89 species) found in the Bay throughout the period of zoobenthos research since 1934 fits into the range of theoretically expected value of the species richness of the polychaete taxocene, approaching it even closer.
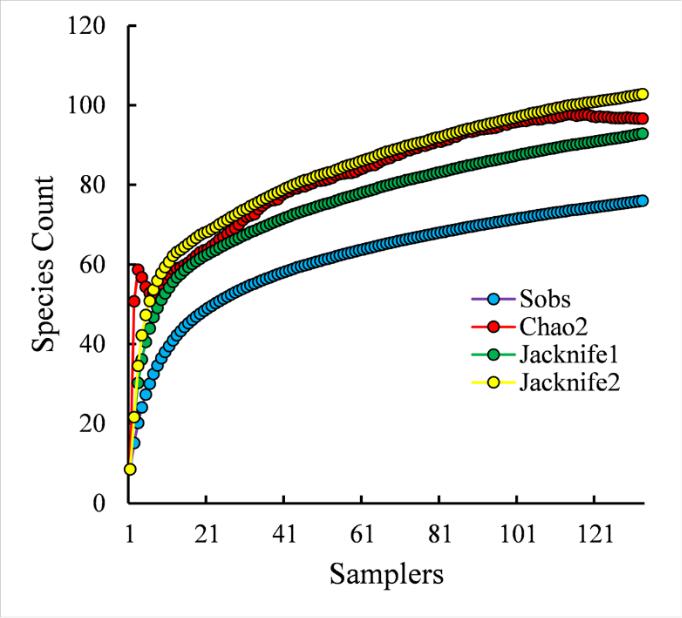
Fig. 5. Prognostic estimation of the species richness of the polychaete taxocene in Karkinit Bay, Black Sea, based on the data of benthos surveys in 2007–2018 Рис. 5. Прогностическая оценка видового богатства таксоцена полихет в Каркинитском заливе, Чёрное море, на основе данных исследований бентоса в 2007–2018 годах
Due to the change in hydrological conditions in the shallow part of the Bay in 2014, caused by the damming of the North Crimean Canal, it is of interest to compare the polychaete taxocene compositions and structures between 2007–2013 and 2016–2018. The similarity index for the faunas of 2007–2013 and 2016–2018 amounted to 0.74 for the Zabakalsky area; for the western deep-water area, 0.77; and in general, 0.83. Such index values indicate that there was a slight change in species composition, a little more pronounced in the Zabakalsky area.
16
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
![]()
In quantitative terms and in the structure of dominance, the differences proved to be more significant. Until 2014, Heteromastus filiformis, Harmothoe imbricata, and Glycera alba were the leading species in the polychaete taxocene in the Zabakalsky area. Then, after the closure of the North Crimean Canal, Platynereis dumerilii and Glycera alba became leading, while H. imbricata and H. filiformis occurred in the category of characteristic species. In 2007–2013, Hediste diversicolor dominated in abundance; in 2016–2018, H. filiformis and P. dumerilii dominated. There were significant differences between complexes I (Heteromastus filiformis + Hediste diversicolor) and IV (Platynereis dumerilii) in terms of the number of polychaetes (p=0.0008). The average density of polychaetes in the period after the closure of the North Crimean Canal decreased 2.7-fold. This was largely due to a decrease in the abundance of H. diversicolor, which averaged at 257±129 ind.·m−2 (with a maximum of 2313 ind.·m−2); in 2018, the average was 10±9 ind.·m−2, and the maximum did not exceed 225 ind.·m−2. This was probably related to the increase in the water salinity in the upper part of the Zabakalsky area (Table 1).
The species H. diversicolor is characteristic of the coastal zone, inhabits silty and silty/sandy sediments, is distinguished by its tolerance to freshening to up to 1.4 ‰, and also survives decreases in oxygen content to 2.44 mL∙L−1. The greatest development of this species was observed in estuaries at a salinity of 5–6 ‰ on silts often contaminated with hydrogen sulfide (Losovska, 1964). During our studies in 2008–2009, the salinity in the shallow-water zone of the Zabakalsky part of the Bay varied from 1.5 to 18.8 ‰; silted sediments with a large amount of detritus and the smell of hydrogen sulfide prevailed. At the stations with a salinity lower than 10 ‰, H. diversicolor dominated, while other polychaetes almost did not occur (Fig. 6a). The H. diversicolor density showed an inverse relationship with the salinity value (Fig. 6b).
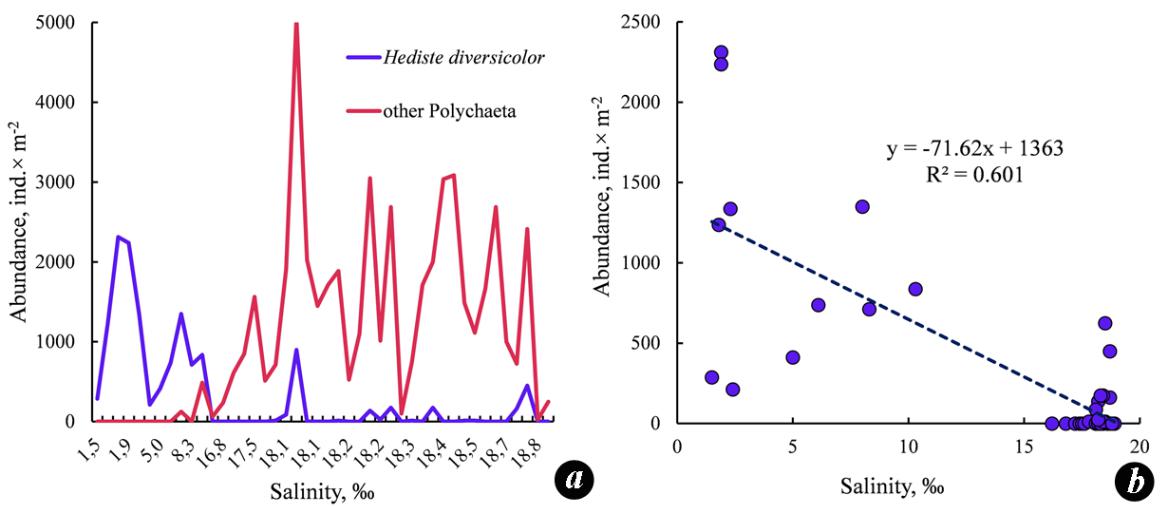
Fig. 6. The density of Hediste diversicolor and other species of Polychaeta in the benthos of
Karkinit Bay at different water salinity values (a) and the relationship of the H. diversicolor density
and the salinity (b) in 2008–2009
Рис. 6. Плотность Hediste diversicolor и других видов полихет в бентосе Каркинитского
залива при различных значениях солености воды (a) и соотношение плотности
H. diversicolor и солености (b) в 2008–2009 годах
At one of the stations, Manayunkia caspica, a species characteristic for brackish waters of the Azov–Black Sea basin, was recorded only during the period of freshening in 2011 (Kiseleva, 2004). M. caspica was found on silty/sandy sediments where it formed a density of 36 ind.·m–2.
In the western area, where the pattern of salinity variations is more stable, no significant interannual differences were observed in the polychaete taxocene. The leading forms were the same species, and the average values of polychaete density were similar: 1420±1293 ind.·m− 2 in 2011– 2013 and 1291±615 ind.·m−2 in 2016–2017. Melinna palmata, H. filiformis and Nephtys hombergii
17
Boltachova N. A., Lisitskaya E. V., Revkov N. K., Podzorova D. V.
![]()
dominated in the former period; Prionospio cf. cirrifera and H. filiformis dominated in the latter period. Differences between assemblages II (Nephtys hombergii + Heteromastus filiformis) and III (Heteromastus filiformis + Prionospio cf. cirrifera + Aricidea claudiae) in polychaete abundance were not significant (p>0.25). By combining the data obtained for the deep-water western area from 2011 to 2017, we composed a map of polychaete density distribution (Fig. 7).
Of particular note are very high values of both the average and maximum densities of M. palmata in 2011–2013 (up to 16740 ind.·m−2 at a depth of 40 m, in the middle part of the Bay) and P. cf. cirrifera in 2016–2017 (up to 2984 ind.·m−2 at a depth of 20 m, in the area of the Small Phyllophora Field). Although similar density values (25000 ind.·m−2) were reported earlier for M. palmata, widely distributed in the NWBS (Losovskaya, 1988; Revkov, Boltachova, 2021), our values of the maximum density of P. cf. cirrifera exceed those known for the Black Sea. Thus, the density of P. cf. cirrifera on sandy sediments off the Crimean coast reached 396 ind.·m−2, while on shell debris/sandy sediments off the coast of Bulgaria, it was 267 ind.·m−2 (Marinov, 1977; Kiseleva, 2004). This species, like many Spionidae, is known to be opportunistic and is characteristic of waters with a high organic matter content (Kiseleva, 2004; Çinar et al., 2009). Therefore, the massive increase in abundance of P. cf. cirrifera that we observed may be associated with the general organic pollution of Karkinit Bay.
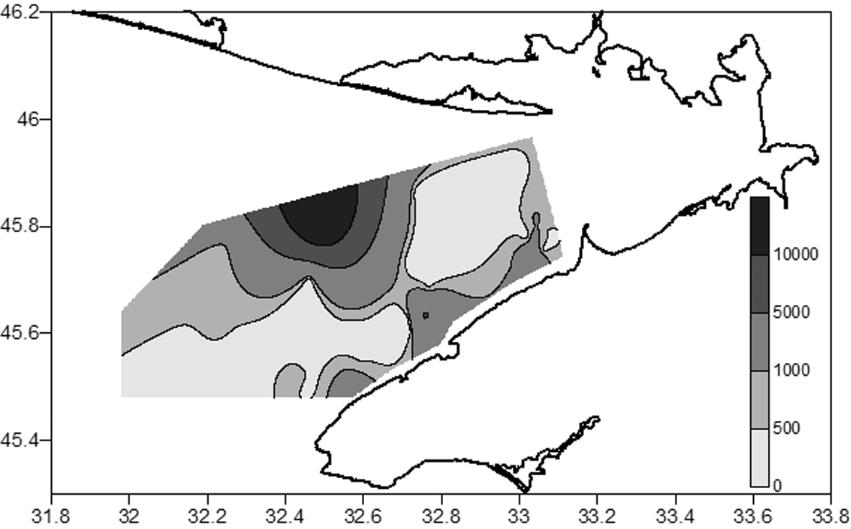
Fig. 7. Distribution of polychaete density (ind.·m−2) in western Karkinit Bay in 2011–2017 Рис. 7. Распределение плотности полихет (экз.·м−2) в западной части Каркинитского залива в 2011–2017 годах
CONCLUSIONS
In 2007–2018, we recorded a total of 75 species of polychaete worms from Karkinit Bay, which allowed us to extend the previously known taxonomic list of polychaetes by adding 46 species. Thus, 86 species of Polychaeta belonging to 32 families were found in Karkinit Bay throughout the period of zoobenthos research from 1934 to 2018. The following families were represented by the largest numbers of species: Phyllodocidae (10 species), Syllidae (11), Spionidae (8), and Nereididae (7).
The average density of polychaetes in the period 2007–2018 varied within a range of 498–1420 ind.·m−2, and the maximum value reached 17708 ind.·m−2. Differences in the structure and abundance of Polychaeta taxocene were observed between the shallow-water Zabakalsky part and the deeper western parts of Karkinit Bay. However, the species Heteromastus filiformis proved to be leading all over the area of the Bay.
The change in hydrological characteristics had an impact on the polychaete taxocene in the shallow waters of the Zabakalsky part of the Bay. In these waters, Hediste diversicolor dominated in
18
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
![]()
abundance, reaching 2313 ind.·m−2, in 2007–2013. This species dominated at stations with a water salinity lower than 10 ‰. The population density of H. diversicolor showed an inverse relationship with water salinity. After the closure of the North Crimean Canal, the dominant species changed, and the average density values of polychaetes decreased 2.7-fold.
In the western, deep-water part, no significant changes have been observed after the closure of the canal. The maximum values of polychaete density were recorded for Melinna palmata (16740 ind.·m−2) in 2007–2013 and for Prionospio cf. cirrifera (2984 ind.·m−2) in 2016–2018.
The lists of Polychaeta species that we have compiled can be used for an inventory of the protected water areas located in the Zabakalsky part of the Karkinit Bay: the Lebyazhy Islands Nature Reserve and the Small Phyllophora Field and Karkinitsky Nature Sanctuary.
Acknowledgments: the work was carried out within the framework of the state task of the Federal Research Center of the IBSS on the topic “Regularities of formation and anthropogenic transformation of biodiversity and bioresources of the Azov-Black Sea basin and other regions of the World Ocean” (registration no. 121030100028-0) and “Study of control mechanisms of production processes in biotechnological complexes in order to develop scientific foundations for obtaining biologically active substances and technical products of marine genesis”, (registration no. 121030300149-0).
The authors are deeply grateful to all colleagues who took part in the collection and processing expedition materials, as well as Dr. N.G. Sergeeva and Dr. I.A. Zhirkov for valuable advice and comments which contributed to improvement of the manuscript.
References
Arnoldy L. V. Materials on the quantitative study of the Black Sea zoobenthos. II. Karkinit Gulf // Trudy Sevastopol’skoj Biologicheskoj Stancii. – 1949. – Vol. 7. – Р. 127–192 [in Russian].
Boltachova N. A., Revkov N. K., Bondarenko L. V., Kolesnikova E. A., Timofeev V. A., Kopiy V. G. Taxonomic composition of macrozoobenthos Karkinitsky Bay (Black Sea) in early XXI century // Marine biological research: achievements and perspectives. – Vol. 2. Tezisy dokladov Mezhdunarodnoy nauchnoy konferentsii, posvyashchonnoy 145-letiyu Sevastopol’skoy biologicheskoy stantsii. (19–24 September 2016). – Sevastopol: ECOSI-Hydrophysics, 2016.– P. 36–39 [in Russian].
Boltachova N. A., Lisitskaya E. V. On the taxonomic classification of Spio (Annelida, Spionidae) species from the Sea of Azov – Black Sea basin // Marine Biological Journal. – 2019. – Vol. 4, N 3. – P. 26–36 [in Russian]. https://doi.org/10.21072/mbj.04.3.03.
Boltachova N. A, Lisitskaya E. V., Podzorova D. V. Distribution of Alien Polychaetes in Biotopes of the Northern Part of the Black Sea // Russian Journal of Biological Invasions. – 2021. – Vol. 12, N 1. – P. 11–26. https://doi.org/10.1134/S2075111721010033.
Bray J. R., Curtis J. T. An ordination of the upland forest communities of South Wiskonsin // Ecological Monographs. – 1957. – Vol. 27. – P. 325–347.
Çinar M. E., Balkis H., Albayrak S., Dağli E., Karhan S. U. Distribution of polychaete species (Annelida: Polychaeta) on the polluted soft substrate of the Golden Horn Estuary (Sea of Marmara), with special emphasis on alien species // Cahiers de Biologie Marine. – 2009. – Vol. 50, N 1. – P. 11–17.
Clarke K. R., Gorley R. M. PRIMER v5: User Manual/Tutorial. Primer–E: Plymouth, 2001. – 92 p.
Foggo A., Attrill M. J., Frost M. T., Rowden A. A. Estimating marine species richness: an evaluation of six extrapolative techniques // Marine Ecology Progress Series. – 2003. – Vol. 248. – P. 15–26.
Gulin S. B., Mirzoeva, N. Yu., Lazorenko G. E., Egorov V. N., Trapeznikov A. V., Sidorov I. G., Proskurnin V. Yu., Popovichev V. N., Bey O. N., Rodina E. A. Present-day radioecological situation, associated with the regime of functioning of the North Crimean Canal // Radiatsionnaya biologiya. Radioekologiya. – 2016. – Vol. 56, N 6. – P. 647–654 [in Russian].
Kiseleva M. I. Polychaetes (Polychaeta) of the Black and Azov Seas. Kola Science Centre, Murmansk Marine Biological Institute, Russian Academy of Science. – Apatity, 2004. – 409 p. [in Russian].
Kondratiev S. I. Hydrochemistry of the northwestern shelf of the Black Sea in the modern period // Sistema Chernogo Morya. [A. P. Lisitsyn ed.]. – Moscow: Scientific World, 2018. – P. 119–146 [in Russian]. https://doi.org: 10.29006/978-5-91522-473-4.2018.119
Losovska G. V. On the distribution of marine species of polychaete Nereis (Neanthes) diversicolor (Müller, 1776) and Nereis (Neanthes) succinea (Leucart, 1847) in the Dnieper-Bug estuary // Naukovi zapiski Odeskoi biologichnoi stantsii. – 1964. – Vol. 5. – P. 34–38 [in Ukrainian].
19
Boltachova N. A., Lisitskaya E. V., Revkov N. K., Podzorova D. V.
![]()
Losovskaya G. V. Long-term changes in the composition and distribution of polychaetes of the north-western part of the Black Sea // Gidrobiologicheskiy Zhurnal. – 1988. – Vol. 24, N 4. – P. 21–25 [in Russian].
Malakhova L. V., Egorov V. N., Gulin S. B., Malakhova T. V., Moseichenko I. N. Long-Term Dynamics of the Concentrations of Organochlorine Compounds and Mercury in the Bottom Sediments of the Chernorechenskoe Reservoir // Water Resources. – 2019. – Vol. 46, N 4. – P. 595–601. https://doi.org/10.1134/S0097807819040146
Marinov T. Bristle worms (Polychaeta). Fauna of Bulgaria. – Vol. 6. – Sofia: Academiae Scientiarium Bulgaricae, 1977. – 258 p [in Bulgarian].
Polikarpov G. G., Lazorenko G. E., Tereshchenko N. N., Korotkov A. A.The contribution of the North Crimean Canal irrigation system to the transfer of cesium, plutonium and americium radionuclides with the Dnieper waters to the Karkinit Bay of the Black Sea // Radioecological response of the Black Sea to the Chernobyl accident. [G. G. Polikarpov, V. N. Egorov eds.]. – Sevastopol: ECOSI-Hydrophysic, 2008. – P. 185–206 [in Russian].
Povchun A. S. Changes in the benthos of the Karkinit Bay over 50 years // Gidrobiologicheskiy Zhurnal. – 1990. – Vol. 26, N 5. – P. 20–27 [in Russian].
Povchun A. S. Changes in the benthic communities of the Karkinit Bay // Mnogoletnie izmeneniia zoobentosa Chernogo moria. [V. E. Zaika ed.]. – Kiev: Naukova Dumka, 1992. – P. 105–138 [in Russian].
Pukhtyar L. D. Seasonal freshening and salinization of waters in the Karkinit Bay // Physical Oceanography. – 2007. – Vol. 17, N 4. – P. 209–222.
Pukhtyar L. D., Ilyin Yu. P., Belokopytov V. N. Seasonal and spatial variability of the thermohaline structure of the Karkinit Bay waters // Ecological Safety of Coastal and Shelf Zones of Sea. – 2003. – Vol. 8. – P. 48–63 [in Russian].
Revkov N. K., Boltachova N. A., Kolesnikova Ye. A., Timofeyev V. A. Macrozoobenthos of the Swan Islands area of the Karkinit Bay (the Black Sea) // Bioraznoobraziye i ustoychivoye razvitiye: Tezisy dokladov mezhdunarodnoy nauchno-prakticheskoy konferentsii. – Simferopol: Krymskij nauchnyj centr NAN Ukrainy i MON Ukrainy, 2010. – P. 108– 111 [in Russian].
Revkov N. K., Boltachova N. A. Structure of the macrozoobenthos assemblages in the central part of the northwestern Black Sea shelf (Zernov’s Phyllophora Field) at the beginning of the 21st century // Ecologica Montenegrina. – 2021. – Vol. 39. – P. 92–108. https://doi.org/10.37828/em.2021.39.11
Sovga E. E., Pasynkov A. A., Andreeva O. A. Ecological state of the coastal regions of Crimea // Ecological Safety of Coastal and Shelf Zones of Sea. – 2011. – Vol. 25. – P. 169–180 [in Russian].
Surugiu V. Systematics and ecology of species of the Polydora-complex (Polychaeta: Spionidae) of the Black Sea // Zootaxa. – 2012. – Vol. 3518, N 1. – P. 45–65.
Terentyev A. S. Distribution of crabs Pilumnus hirtellus (Decapoda, Xanthidae) in a small phyllophora field in Karkinitsky Bay of the Black Sea // Vestnik zoologii. – 2002. – Vol. 36, N 5. – P. 69–72 [in Russian].
Vinogradov K. O. On the issue of feeding area of bottom fish in the northwestern part of the Black Sea // Naukovі zapiski Odes’koї bіologіchnoї stancії. – 1959. – Vol. 1. – P. 98–111 [in Ukrainian].
Vinogradov K. A., Losovskaya G. V. Class polychaete worms – Polychaeta // Opredelitel’ fauny Chernogo i Azovskogo morey. – Vol.1. [F. D. Mordukhai-Boltovskoy ed.]. – Kiev: Naukova Dumka, 1968. – P. 251–359 [in Russian].
Vodyanitsky V. A. On the natural-historical zoning of the Black Sea and, in particular, off the coast of Crimea // Trudy Sevastopol’skoj biologicheskoj stancii. – 1949. – Vol. 7. – P. 249–255 [in Russian].
Vorobyov V. P. Benthos of the Sea of Azov // Trudy Azovsko Chernomorskogo nauchno issledovatel’skogo instituta morskogo rybnogo hozyajstva i okeanografii. – Simferopol: Krymizdat, 1949. – Vol. 13. – 193 p. [in Russian].
Yakubova L. I. List of Archiannelides and Polychaeta of the Sevastopol Bay of the Black Sea // Izvestia Akademii Nauk SSSR. – 1930. – Vol. 7. – P. 863–881 [in Russian].
Zakuts’kij V. P. On the question of reserve of zoobenthos in the northwestern part of the Black Sea // Doklady Akademii Nauk URSR. – 1962. – Vol. 10. – P. 1376–1377 [in Ukranian].
Zakutsky V. P., Vinogradov, K. A. Macrozoobenthos // Biology of the northwestern part of the Black Sea. [K. A.
Vinogradov ed.]. – Kiev: Naukova dumka, 1967. – P. 146–157 [in Russian].
Zernov S. A. To the issue of the study of the Black Sea life // Notes of the Imperial Academy of Sciences. Ser. 8. – St. Petersburg, 1913. – Vol. 32, N 1. – 299 p. [in Russian].
Zolotarev P. N., Povchun A. S. Macrozoobenthos of the deep-water zone of the Karkinit Bay // Ekologiya moray. – 1986. – Vol. 22. – P.48–58 [in Russian].
Zolotarev P. N., Rubinshtejn I. G., Larchenko N. A., Povchun A. S. The state of the benthos of Karkinit Bay in the Black Sea in the 1980s. – Sevastopol’: Institut biologii yuzhnykh morey AN USSR. N 5447. Dep. V VINITI. – 1991. – 34 p. [in Russian].
Yurovsky Yu. G. Study of the coast-sea system in the North Western Crimea // Ecological Safety of Coastal and Shelf Zones of Sea. Sevastopol’: ECOSI-Hydrophysics. 2001. – P. 154–165 [in Russian].
20
Polychaetes in benthos of Karkinit Bay, northwestern Black Sea
![]()
Болтачева Н. А., Лисицкая Е. В., Ревков Н. К., Подзорова Д. В. Полихеты в бентосе Каркинитского залива (Чёрное море, северо-западная часть) // Экосистемы. 2022. Вып. 30. С. 5–21.
-
- результате исследований макрозообентоса, выполненных в 2007–2018 годах в Каркинитском заливе Чёрного моря, зарегистрировано 75 видов Polychaeta. Известный ранее таксономический список полихет дополнен 46-ю новыми видами. Установлено, что за весь период исследований зообентоса (1930–2018 годы) в Каркинитском заливе обнаружено 86 видов Polychaeta, относящихся к 32 семействам. По числу видов наиболее широко представлены семейства Phyllodocidae – 10, Syllidae – 11, Spionidae – 8, Nereididae – 7 видов. В период наших исследований средняя плотность полихет варьировала в пределах 498–1420 экз.∙м–2, максимальная достигала 17708 экз.∙м−2. Отмечены различия в структуре и количественном развитии Polychaeta в мелководной Забакальской и в более глубоководной западной частях Каркинитского залива. На таксоцен мелководных участков Забакальской части залива существенное влияние оказало повышение солености, обусловленное прекращением действия Северо-Крымского канала в Крыму (2014 год). В 2007–2013 годах на станциях с соленостью менее 10 ‰ по численности преобладал Hediste diversicolor, достигая 2313 экз.∙м−2. Отмечена обратная зависимость плотности H. diversicolor от величины солености. Показано, что в 2016–2018 годах в Забакальской части залива произошла смена доминирующих видов, а средняя плотность полихет уменьшилась в 2,7 раза. В западной глубоководной части Каркинитского залива существенных изменений после прекращения работы канала не зарегистрировано. Максимальные показатели плотности (16740 экз.∙м−2) полихет отмечены у Melinna palmata в 2007–2013 годах, а в
2016–2018 годах – у Prionospio cf. cirrifera (2984 экз.∙м−2). Вид Heteromastus filiformis указан как руководящий во всей акватории залива.
Ключевые слова: Annelida, Polychaeta, Hediste diversicolor, зообентос, Чёрное море.
Поступила в редакцию 15.07.22
Принята к печати 02.08.22
21

